Research Article - Biomedical Research (2017) Volume 28, Issue 2
Antimicrobial activity and Chemical analyses of oil constituents of Medicinal Plant Costus speciosus (Koen.)
Manal Othman Al-Kattan1 and Suzan A. Khayyat2,3*1Department of Microbiology, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
2Department of Chemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
3Faculty of Science and Arts in Rabigh, King Abdulaziz University, Jeddah, Saudi Arabia
- *Corresponding Author:
- Suzan A. Khayyat
Department of Chemistry
Faculty of Science for Girls
King Abdulaziz University
Saudi Arabia
Accepted on June 20, 2016
Abstract
Costus speciosus is a unique Indian ornamental plant species in the genus Costus that has used in traditional medicine where exhibited high importance to multi-purpose pharmacological applications. Essential oil was first obtained from Costus speciosus dried roots, ethanolic extract containing diosgenine was isolated and epoxidized by using m-chloroperbenzoic acid to yield diosgenine epoxide, then analyzed them by using IR, NMR and GC-MS methods. Antimicrobial activities of the essential oil, organic extracts of the dried roots of Costus speciosus were scored on the basis of the change in the general shape of the fungal spores SEM analysis. The results showed that in vitro treatment of fungi by the essential oil led to hyphae disruptions and changes in the fungal spore shapes. The studies on the antimicrobial activity showed high growth inhibition for Microsporum gypseum, M. canis, Candida albicans, Pseudomonas aeruginosa and Staphylococcus aureus when challenged with concentrated oil extract. Methanol and ethanol extracts of the oil exhibited an inhibitory effect on the fungi, yeast and bacteria, while the constituent diosgenin epoxide ingredient in the oil showed higher in vitro growth inhibitory activity than diosgenin against M. gypseum, C. albicans and C. tropicalis.
Keywords
Medicinal plants, Dermatophytes, Antimicrobial activity, Costus speciosus (Koen.), m-chloroperbenzoic acid, Epoxidation, Diosgenine.
Introduction
Plants have been an important source of antibiotics for thousands of years. The World Health Organization (WHO) has estimated that up to 80% of people worldwide still rely mainly on traditional remedies such as herbs for their medicines. Plants are also alternatives to antibiotics and have fewer side effects compared to allopathic medicine [1]. The Zingiberaceae family constitutes a vital group, medicinal and aromatic plants which characterized by the presence of volatile oils and oleoresins of export value [2]. The rhizomes and roots of Costus speciosus are characterized as bitter, astringent, purgative, anthelmintic, antioxidant, antitumor improves digestion and stimulant [2-6]. Juice of the rhizome is used to relive from headache and for cooling [3,7].
On the other hand, dermatophytes cause some of the most important diseases that infect humans and animals, particularly affecting keratinous tissue such as skin, hair and nails. Trichophyton, Microsporum, and Epidermophyton are the three major groups of dermatophytes, and these microorganisms are related to tinea and ringworm infections [8,9]. These microorganisms are characterized by easy transmission between hosts, wide prevalence and presence in large human and animal reservoirs [10]. Examination of 1254 human patients and isolated several samples from their skin, hair and nails and identified various dermatophytes, such as T. rubrum, T. mentegrophytes, M. gypseum and M. canis, all of these dermatophytes are associated with a type of tinea [11]. Most human skin infections are caused by a fungi of the type Microsporum, the study [12] analyzed M. canis from 944 patients who visited an outpatient clinic over a 17-year period (1993-2002) in Korea. In addition, Cutaneous candidiasis is an opportunistic infection and isolated Candida species from 250 diverse clinical sources [13]. The C. albicans (39.64%) was the most frequently isolated species on mucosal surfaces and superficial infections of the skin [14]. Bactria is found that “green nail syndrome” a type of skin lesions caused by P. aeruginosa, and it is highly associated with tinea pedis patients [15]. The macerated interspaces pathogen defined were S. aureus, Brevibacterium epidermidis and Pseudomonas species [16]. Mixed fungal and bacterial infections were seen in deep wounds of diabetic patients and included C. albicans, C. tropicalis, Enterococcus faecalis, S. aureus and P. aeruginosa. Most of these fungi and bacteria were resistant to antibiotics such as itraconazole, amphotericin B, voriconazole and flucytosine [17,18].
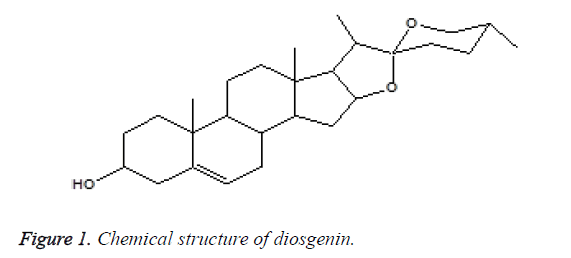
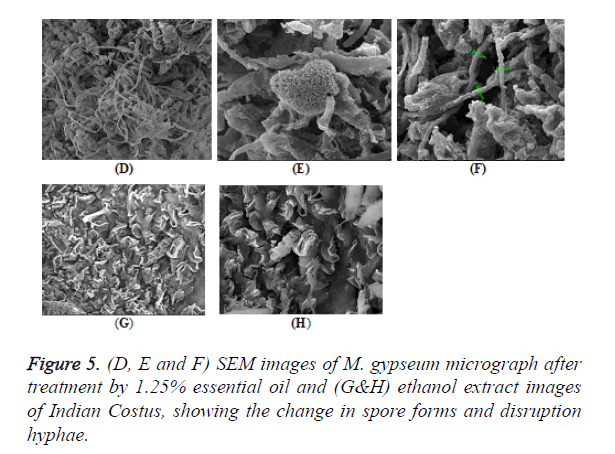
Extracted essential oil and diosgenin (Figure 1), a steroidal sapognein from Costus plants has been previously demonstrated for its estrogenic effects and possible pharmaceutical exploitation [19]. The aim of this work is to ascertain the antimicrobial effects of dried fine powder of Indian Costus plant roots. Epoxidation of diosgenin was performed by using m-chloroperbenzoic acid. The extracts, essential oil, diosgenin were tested for their antimicrobial activity. After treatment with essential oil extract, the change in the general shape of the fungal spores was examined by SEM analysis.
Material and Methods
Chemistry
IR spectra were performed on a Perkin-Elmer 16 FPC FT-IR spectrophotometer as thin films. 1HNMR and 13CNMR spectra were obtained in CDCl3 solution with a Bruker AVANCE D.P.X. 600 MHz apparatus. GC-MS were determined by Joel JMS 600H, GC Hewlett Packerd, HP 6890 Series, with a capillary column (30 m, 0.32 nm, 0.25 ml) HP-5 cross linked 5% dimethyl polysiloxane. The change in the general shape of the fungal spores after treatment with essential oil extract was examined with Scanning Electron Microscopy in King Fahd Center for Medical Research-King Abdulaziz University- Jeddah, Saudi Arabia. Thin layer chromatography (TLC) and Preparative Layer Chromatography (PLC) were done by Polygram SIL G/W 254, Mecherey-Nagel. A rotator evaporator (at 20°C/15 torr) was used to remove the solvents.
Plant material
The dried roots of Indian Costus were collected from herb store (Jeddah, Saudi Arabia). All the roots were washed with water, dried and ground well. The fine powder obtained was used to extract the essential oil and this oil was tested for its antimicrobial activity [20].
Extraction of essential oil
The dried root fine powder (550 g) of Indian Costus was subjected to steam distillation for three hours. About 50 mL of the distillates were collected and extracted with chloroform (3 × 100 mL). The extracts were dried over anhydrous sodium sulphate (Merck, Germany), and the solvent was removed by evaporation. The essential oil yield obtained was around 0.65% and was stored in a refrigerator at 6°C.
Preparation of plant extracts
The dried powdered roots of Indian Costus (200 g) was extracted successively using cold percolation system of ethanol, methanol, distilled water or chloroform (400 ml for each) for 4 days, using a stirring apparatus [21]. The solutions collected were filtered through Whatman filter paper. The extracts were evaporated to dryness under reduced pressure at 90°C by Rotary vacuum evaporator to obtain the respective extracts and stored under liquid nitrogen.
General epoxidation procedure of natural steroids using m-chloroperbenzoic acid
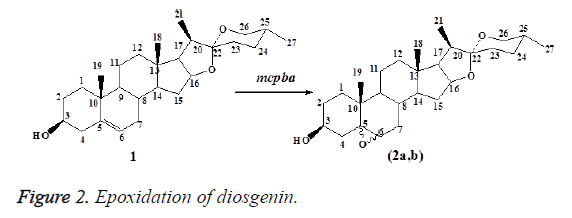
A solution of m-CPBA (10 mmol, 80%) was added cautiously drop wise over 15 min to a stirred solution 1 (5 mmol) in CHCl3 (25 ml) at 0°C. The mixtures were stirred continuously in an atmospheric nitrogen at room temperature (TLC, peroxide test by KI, 10%), after which it was carefully washed with a saturated aqueous solution of NaHCO3 (3 × 10 ml) followed by distilled water (3 × 10 ml). The organic layers were separated, dried over anhydrous Na2SO4 and evaporated under reduced pressure at room temperature. The crude residue products were purified by column chromatography on silica gel adsorbent. Elution of the column was performed with the solvent mixture of petroleum ether 60-80°C and ether (9:2), which gave the epoxide product 2a,b.
Diosgenin (1) (3β,25R)-spirost-5-en-3-ol): Colorless crystal, mp: 204-206°C, C27H42O3 (M. wt., 414.6). IR (KBr) cm-1: 3417 (br, OH), 2933 (CH, s), 1602 (C=C), 1169 (C-O). 1HNMR (CDCl3): δ 0.79 (d, 3H, J=6 Hz, H-27), 0.80 (s, 3H, H-18), 0.98 (d, 3H, J=7 Hz, H-21), 1.02 (s, 3H, CH-19), 2.34 (comp. pat., 2H, H-7) 3.38 (dd, 1H, J=11 Hz, H-26), 3.47 (dd, 1H, J=11, 4 Hz, H-26), 3.52 (comp. pat., 1H, H-3), 4.42 (dd, 1H, J=11, 7 Hz, H-16), 5.38(d, 1H, J=5 Hz, H-6), 0.79-2.31 (comp. pat., remaining protons). 13C-NMR, DEPT (CDCl3) δ: 14.5 (C21), 16.3 (C27), 17.2 (C19), 19.4 (C18), 20.9 (C11), 28.8 (C15), 30.3 (C8), 31.4 (C25), 31.5 (C24), 31.6 (C7), 31.9 (C12), 32.1 (C1), 36.7 (C13), 37.2 (C23), 39.8 (C2), 40.3 (C1°), 41.6 (C2°), 42.3 (C4), 50.1 (C14), 56.5 (C9), 61.9 (C17), 66.9 (C26), 71.8 (C3), 80.8 (C16), 111.9 (C22), 121.4 (C6), 140.8 (C5). MS: m/z 414 (M+, C27H42O3, 3%), 353 (M+-C3H9O, 3%), 342 (M +-C4H8O, 8%), 300 (M+ C6H10O2, 11%), 267 (M+-C8H19O2, 12%), 139 (C9H15O, 100%).
5,6-Epoxyspirostane (2a,b): Colorless crystal, mp: 195-200°C, C27H42O4 (M. wt., 430.6). IR (KBr) cm-1: 3383 (br, OH), 2926, 2869(CH, s), 1601 (C=C), 1101 (C-O). MS: m/z 430 (M+, C27H42O4, 5%), 358(C24H38O2, 10%), 325 (C22H29O2, 5%), 139 (C9H15O, 100%).
Exo form 2a (5α,6α-Epoxyspirostane): 1H-NMR (CDCl3): δ 0.75 (s, 3H, H-18), 0.81 (d, 3H, J=6 Hz, H-27), 0.98 (d, 3H, J=7 Hz, H-21), 1.1 (s, 3H, H-19), 2.93 (d, 1H, J=4 Hz, H-6) 3.38 (dd, 1H, J=11 Hz, H-26), 3.5 (dd, 1H, J=11, 3 Hz,H-26), 3.93 (comp. pat., 1H, H-3), 4.41 (dd, 1H, J=7.5 Hz, H-16), 0.71-2.11 (comp. pat., remaining protons). 13C-NMR, (CDCl3): δ 14.5 (C21), 16.3 (C27), 17.2 (C19), 19.4 (C18), 20.8 (C11), 28.8 (C15), 30.3 (C8), 31.4 (C25), 31.4 (C24), 31.6 (C7), 31.8 (C12), 32.2 (C1), 36.6 (C13), 37.2 (C23), 39.9 (C2), 40.4 (C1°), 42.1 (C17), 42.3 (C4), 41.6 (C2°), 50.0 (C14), 56.5 (C9), 61.8 (C6), 62.1 (C16), 65.2 (C3), 66.9 (C26), 71.7 (C5), 111.8 (C22).
Endo form 2b (5β,6β-Epoxyspirostane): 1H-NMR (CDCl3): δ 3.11 (d, 1H,J=4 Hz, H-6), 4.26 (comp. pat., 1H, H-3), remaining protons appeared as 2a. 13C-NMR (CDCl3): δ 14.4 (C21), 16.2 (C27), 17.2 (C19), 19.4 (C18), 20.6 (C11), 28.8 (C15), 30.3 (C8), 31.4 (C25), 31.4 (C24), 31.6 (C7), 31.8 (C12), 32.2 (C1), 36.0 (C13), 37.2 (C23), 40.0 (C2), 40.4 (C1°), 42.1 (C17), 42.3 (C4), 41.6 (C2°), 50.1 (C14), 56.6 (C9), 61.9 (C6), 62.2 (C16), 65.2 (C3), 66.9 (C26), 71.6 (C5), 111.8 (C22).
Antimicrobial activities
Test for Fungi and yeast pathogenic: The M. gypseum, M. canis, C. albicans and C. tropicalis isolates were obtained from King Faisal Specialist Hospital & Research Centre-Jeddah, Saudi Arabia. They were cultured on sabaroud dexterous agermedia (Oxoid CM 41) at 25°C.
Bacterial pathogenic: P. aeruginosa and S. aureus were obtained from King Faisal Specialist Hospital & Research, Centre-Jeddah, Saudi Arabia. A blood agar a media (Oxoid) was used for cultivation of pathogenic bacteria at 37°C [22].
Antimicrobial activities of essential oils and plant extracts of Indian Costus: About 0.5 g of the testing compound was dissolved in 1.25 ml of a solvent (chloroform, ethanol and methanol) and it was cultured in sabaroud dexterous agar media and blood agar media using the agar disc diffusion method. Each media used were inoculated with 1 ml from a suspension of M. gypseum, M. canis and C. albicans for sabaroud dexterous agar and P. aeruginosa and S. aureus for blood agar. The fungi and yeast were incubated at 25°C for 8 days, and 48 hours respectively and then tested at 37°C for 24 hours. After that, the diameter of inhibition zones was measured in millimeters [23,24].
Results and Discussion
The curative properties of medicinal plants are perhaps due to the presence of various secondary metabolites such as alkaloids, flavonoids, glycosides, phenols, saponins, sterols etc. The extracts of rhizomes have revealed the presence of alkaloids, flavonoids, cardiac glycosides, saponins, sterols and tannins, which may have led to their discovery and development as drugs [25]. The pharmacological action of crude extract is determined by the nature of their constituents [26]. The aqueous extract of Costus speciosus rhizomes showed antimicrobial activity against Staphylococcus aureus. However, the organic phase (e.g. methanolic extract) did not show inhibitory activity against any bacteria [25]. The steroidal sapogenine diosgenine (1) has been successfully isolated from C. specious (Koen) rhizome extract and identified with authentic sample’s by comparison of spectroscopic data.
The epoxidation of diosgenin (1) using m-chloroperbenzoic acid (m-CPBA) at room temperature gave the regioisomeric 5α,6α-epoxyspirostane and 5β,6β-epoxyspirostane (2a,b) (exo and endo forms) in 72% yield (Figure 2). Chemical structures were confirmed through spectral analysis, 1H-NMR, 13C-NMR and MS spectrums which were in a good agreement with [19].
1H-NMR spectrum of 2a showed doublet of doublet at δ 2.93 for H-6 which was shifted from δ 5.36 of starting 1. 13C-NMR spectrum of 2a showed signals at δ 71.7 and 61.9 for carbons in positions 5 and 6. 1H-NMR spectrum of 2b showed doublet at δ 3.11 for H-6 and complex pattern at 4.21 for H-3. 13C NMR spectrum of 2b showed signals at δ 71.6 and 61.9 for carbons in positions 5 and 6 which were shifted from 140.8 and 121.4 of the same carbons of the start 1. MS spectrum of 2a showed molecular ion at m/z 430 for its molecular weight.
Antimicrobial activities of essential oils and plant extracts of Indian Costus
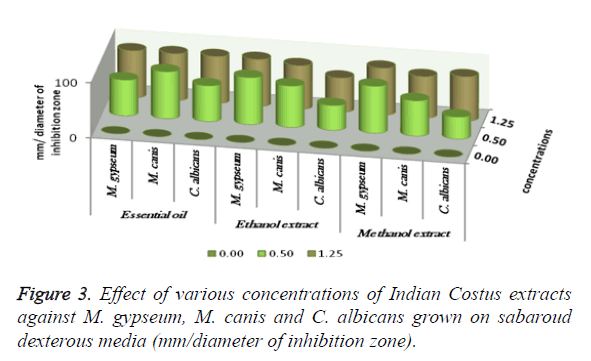
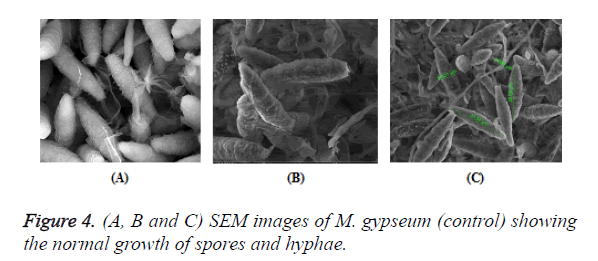
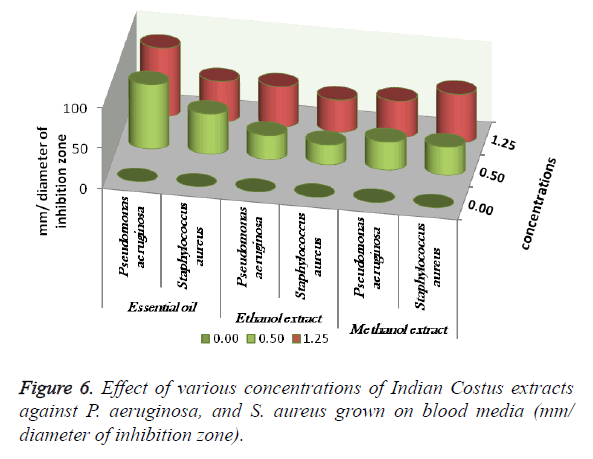
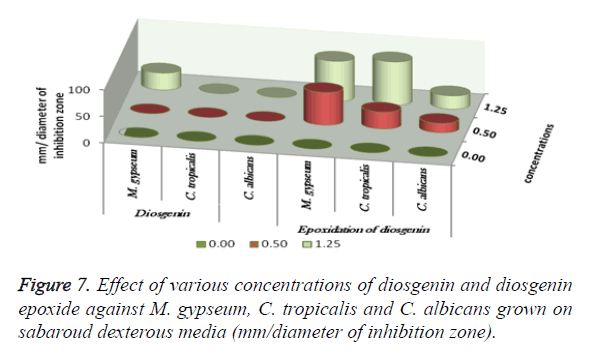
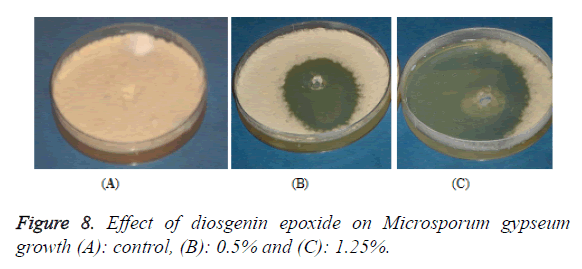
The antimicrobial activities of Indian Costus extracts were tested on fungi, yeast and bacteria. The results (Figure 3) showed high inhibition of M. gypseum, M. canis and C. albicans. The treatment by oil extract showed an inhibitory effect on the fungi with methanol and ethanol extracts. At 1.25 m the inhibition zone was 85 mm of essential oil, (85, 75 and 80 mm) of methanol extract and (85, 79 and 62 mm) of ethanol extract respectively. In addition, M. gypseum growth was more sensitive than M. canis and C. albicans at 1.25 m of all extracts compared to the control sample. The SEM images obtained for the essential oil efficiency on M. gypseum hyphae and spores showed that treatment with the essential oil led to hyphae disruptions and changes in the general shape of the fungal spores (Figures 4 and 5). The same extract concentrations of Costus on pathogenic bacteria showed that the growth of P. aeruginosa was more affected by essential oil than the ethanol and methanol extracts. On the other hand, the inhibition zone measurements were 60 and 50 mm for methanol and essential oil on S. aureus, whereas the Costus methanolic extract was very effective on these bacteria (Figure 6). Indian Costus diosgenin was effective against M. gypseum at 1.25 m. However, it was not as effective against C. tropicalis and C. albicans growth when used in the same extract concentrations. Further, the epoxidation of diosgenin resulted in more activity than the diosgenin extract against the fungus and yeasts (Figures 7 and 8) where, the results for the inhibition zone measurements were 76, 82 and 25 mm compared with the control sample.
Based on previous reports, the Indian Costus roots have several of medicinal components items that are used in the treatment of several diseases. Saussurea costus is a popular medicinal plant, with sesquiterpene lactones as major phyto constituents and contains other pharmacological compounds such as costunolide, dehydro costus lactone and cynaropicrin which exhibit anti-inflammatory, anti-ulcer, anticancer and hepatoprotective activities in vitro and in vivo models [27]. Reports by [28,29] have shown the antioxidant activity of the medicinal plant Costus speciosus which has been used for diabetic males by treatment of costunolide and eremanthin, those compounds are isolated from this plant by gas chromatography-mass spectrometry (GC-MS) analysis. However, the antibacterial and antifungal activities were very high with by dichloromethane and methanol extracts, which were isolated from species of Alpinia, Costus and Zingiber [30]. The essential oil, methanol and ethanol extracts of Costus roots contain an antimicrobial activity, and methanolic and aqueous extracts of Costus arabicus one of 12 Yemeni herbs that are used as traditional medicine for human cancer cells, have demonstrated an inhibitory effect against gram-positive, three gram-negative bacterial and one fungal stains as well as three resistant Staphylococcus strains [31].
The results obtained from [32,33] revealed the efficiency of the dried roots and aquatic extracts (hot and cold) of Indian Costus used in high concentrations against Aspergillus niger, A. flavus, A. fumigatus and C. albicans. Concentrations of 20%, 25% and 30% were highly effective against the tested fungus, yeast and bacteria, which are respiratory pathogens. Thus, as the previous results indicate the roots of Indian Costus species contain several chemical compounds that are curative for dermatophyte infections. They can be used as an alternative to antibiotics, since they show a double effectiveness insert (antifungal and antibacterial). Thus, this study opens up the possibility of working with therapeutic medical products derived from the roots of Indian Costus.
Conclusion
The antimicrobial activities of Indian Costus extracts were tested on fungi, yeast and bacteria. Fine powder from the dried roots of Indian Costus was used to extract the essential oil. Epoxidation of the extract was performed with mchloroperbenzoic acid with CHCl3 in a nitrogen atmosphere. The extracts and essential oil were tested for their antimicrobial activity. After treatment with essential oil extract, the change in the general shape of the fungal spores was examined by SEM analysis. The results showed that treatment by the essential oil led to hyphae disruptions and changes in the general shape of the fungal spores. The studies on the antimicrobial activity showed high growth inhibition in M. gypseum, M. canis, C. albicans, P. aeruginosa and S. aureus. Further, treatment by oil extract showed greater inhibitory effects on the fungi and yeast than the methanol and ethanol extracts. In addition, the epoxidation of diosgenin resulted in more activity than diosgenin extract against the fungus and yeasts.
References
- Kala CP, Dhyani PP, Sajwan BS. Developing the medicinal plants sector in northern India: challenges and opportunities. J Ethnobiol Ethnomed 2006; 2: 32-37.
- Nahak G, Kantasahu R. Free radical scavenging activity of rhizome of Costus Speciosus (KOEN) J. E. SM. Int J Ins Pharm and L Sci 2011; 1: 62-67.
- Gupta RK. Medicinal and Aromatic Plants with Colour Plates: Traditional and Commercial Uses Agrotechniques Biodiversity Conservation (HB) 2010; CBS: 234-499.
- Nadkarni KM. Indian Materia Medica. Bombay Popular Prakashan, India, 2009; 385-386.
- Vijayalakshmi MA, Sarada NC. Screening of Costus speciosus extracts for antioxidant activity. Fitoterapia 2008; 79: 197-198.
- Deni B. Encyclopaedia of Herbs, The Royal Horticulture Society 2008; P: 181.
- Srivastava S, Singh P, Mishra G, Jha KK, Khosa RL. Costus specious (keukand): a review. Der Pharmacia Sinica 2011; 2: 118.
- Al Hasan MS, Fitzgerald M, Saoudian M, Krishnaswamy G. Dermatology for the practicing allergist: Tinea pedis and its complications. Clin Mol All 2004; 2: 7961-7965.
- Mukherjee PK, Isham N, Ghannoum MA. Infectious Diseases of the Skin: Dermatophytosis/Onychomycosis. Curr Clin Pathol Mol Diagnos Dermatol Dermatopathol 2011.
- Dahdah MJ, Scher RK. Dermatophytes. Curr Fungal Infect Rep 2008; 2: 81-86.
- Falahati M, Akhlaghi L, Rastegar Lari A, Alaghehbandan R. Epidemiology of dermatophytoses in an Area South of Tehran, Iran. Mycopathol 2003; 156: 279-287.
- Lee JW, Song CH, Lee SD, Kim WJ, Jun B, Bang YJ. Decreasing Prevalence of Microsporum canis Infection in Korea: Through Analysis of 944 Cases (1993-2009) and Review of Our Previous Data (1975-1992). Mycopathol 2012; 173: 235-239.
- Mohandas V, Ballal M. Distribution of Candida Species in Different Clinical Samples and Their Virulence: Biofilm Formation, Proteinase and Phospholipase Production: A Study on Hospitalized Patients in Southern India. J Glob Infect Dis 2011; 1: 4-8.
- Raz-Pasteur A, Ullmann Y, Berdicevsky I. The Pathogenesis of Candida Infections in a Human Skin Model: Scanning Electron Microscope Observations. ISRN Dermatol 2011.
- Bisno AL. Cutaneous infections: microbiologic and epidemiologic considerations. J Am Med 1984; 76: 172-179.
- Kates SG, Nordstrom KM, McGinley KJ, Leyden J. Microbial ecology of interdigital infections of toe web spaces. J Am Acad Dermatol 1990; 22: 578-582.
- Citron DM, Goldstein EJ, Vreni Merriam C, Lipsky BA, Abramson MA. Bacteriology of Moderate-to-Severe Diabe c Foot Infections and In Vitro Activity of Antimicrobial Agents. J Clin Microbiol 2007; 45: 2819-2828.
- Chellan G, Shivaprakash SS, Ramaiyar K, Varma AK, Varman N, Sukumaran MT, Vasukutty JR, Bal A, Kumar H. Spectrum and Prevalence of Fungi Infecting Deep Tissues of Lower-Limb Wounds in Patients with Type 2 Diabetes. J Clin Microbiol 2010; 48: 2097-2102.
- Elgendy E, Al-Ghamdy H. Thermal and Photooxidation Reactions of the Steroids: β Sitosterol, Stigmasterol and Diosgenin. J Taiwan Pharm 2007; 59: 113-132.
- Ody P. The herb society's complete medicinial herbal. Translation: Elvira Academic International 1999; 118-120.
- Duraipandiyan V, Ignacimuthu S. Antibacterial and antifungal activity of Flindersine isolated from Toddalia asiatica (L) Lam. A traditional medicinal plant. J Ethnopharmacol 2009; 123: 494-498.
- Madigan M, Martinko J. Brock biology of microorganisms, 11th ed. Prentice Hall. ISBN 2005; 0131443291.
- Baker FJ, Breach MR. Medical Microbiological Techniques, Butterworths 1980.
- Hasenekoglu H. Laboratory techniques for micro fungi. Ataturk University. Erzurum 1990.
- Saraf A. Phytochemical and Antimicrobial Studies of Medicinal Plant Costus Speciosus (Koen.). E J Chem 2010 ; 7: S405-S413.
- Mukherjee PK. Quality Control of Herbal Drugs, an approach to evaluation of botanicals.1st ed. Business Horizons 2002; New Delhi.
- Pandey MM, Rastogi S, Rawat AK. Saussurea costus botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J Ethnopharmacol 2007; 110: 379-390.
- Eliza J, Daisy P, Ignacimuthu S, Duraipandiyan V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm., in STZ-induced diabetic rats. Chem Biol Interact 2009; 1: 67-72.
- Eliza J, Daisy P, Ignacimuthu S. Antioxidant activity of costunolide and eremanthin isolated from Costus speciosus (Koen ex. Retz) Sm. Chem Biol Interact 2010; 3: 467-472.
- Habsah M, Amran M, Mackeen MM, Lajis NH, Kikuzaki H, Nakatani NA, Rahman A, Ali AM. Screening of Zingiberaceae extracts for antimicrobial and anti-oxidant activities. J Ethnopharmacol 2000; 72: 403-410.
- Mothana RA, Gruenert R, Bednarski PJ, Lindequist U. Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Pharmazie 2009; 4: 260-268.
- Al-Kattan MO. Effect A-wazarin2 preparation from camel's urine on some pathogenic Bactria for digestive system. PhD Thesis 2006; 60-68.
- AL-Kattan MO, AL-Sheikh HM. Effect of water extract of Indian Costus or sea-Qust on pathogenic fungi for the respiratory system in human to exhibit the miracle scientific in the Sunah. Ass Univ Bull Environ Res 2011; 14: 1-14.