Research Article - Biomedical Research (2017) Volume 28, Issue 1
Antimicrobial activities of some newly synthesized substituted Benzosuberone and its related derivatives
Osama I Abd El-Salam1, Ali S Alsayed2, Korany A Ali1, Ahmed A Abd Elwahab1, Abd El-Galil E Amr1,3*, Ghada EA Awad41Applied Organic Chemistry Department, National Research Centre, Dokki, Giza 12622, Egypt
2Chemistry Department, Al-Azhar University, Nasr City, Cairo 1435, Egypt
3Department of Pharmaceutical Chemistry, Drug Exploration & Development Chair, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia
4Chemistry of Natural and Microbial Products Department, Pharmaceutical Industries Division, National Research Centre, 33 El Buhouth St. (Former El Tahrir St.), Dokki, Giza 12622, Egypt
- *Corresponding Author:
- Abd El-Galil E Amr
Department of Pharmaceutical Chemistry
Drug Exploration & Development Chair College of Pharmacy
King Saud University Saudi Arabia
Accepted on May 10, 2016
Abstract
A series of substituted benzosuberone scaffold fused with pyrazole, isoxazole, pyrimidine and triazole moieties were synthesized and evaluated as antimicrobial agents. Some reactions were performed under microwave irradiation, resulted in short time reactions and surprisingly high yields. Some of the obtained products showed remarkable antimicrobial activities and the most active products 7b, 9, 13 and 14 were further tested to evaluate their Minimum Inhibitory Concentration (MIC) value at 125 μg/mL. The structures of new compounds were proved by their elemental analyses and spectral data.
Keywords
Substituted benzosuberone, Pyrimidine, Pyrazole, Triazole, Antimicrobial activity.
Introduction
According to World Health Organization, the continuous use of antibiotics in treating infections has led to the emergence of Multidrug Resistance (MDR) among the various strains of microorganisms [1,2]. The important class of pyrazoles have been reported to be of broad antimicrobial [3-5], antitubercular [6], antiviral [7] and anticancer [8,9] activities. Their activities to treat inflammation, convulsion, and depression were also published [10-13]. In light of the aforementioned findings and our interest in the synthesis of novel heterocycles of biological importance [14-20], we have synthesized some new fused pyrazole, isoxazole and triazol moieties with benzosuberone and 3-nitrobenzosuberone to evaluate their anticipated antimicrobial activities.
Material and Methods
Chemistry
All melting points were measured on a Gallenkamp melting point apparatus (Weiss Gallenkamp, London, UK). The infrared spectra were recorded in potassium bromide disks on a PyeUnicam SP 3300 and Shimadzu FT IR 8101 PC infrared spectrophotometers (PyeUnicam Ltd. Cambridge, UK and Shimadzu, Tokyo, Japan, respectively). The NMR spectra were recorded on a Varian Mercury VX-300 NMR spectrometer (Varian, Palo Alto, CA, USA). 1H spectra were run at 300 MHz and 13C spectra were run at 75.46 MHz in dimethylsulfoxide (DMSO-d6) (Sigma-Aldrich, St. Louis, MO, USA). Chemical shifts are given in parts per million and were related to that of the solvent. Mass spectra were recorded on a Shimadzu GCMS-QP 1000 EX mass spectrometer (Shimadzu, Tokyo, Japan) at 70 eV. Microwave irradiations were carried out in a StartSYNTH Microwave apparatus (ATS Scientific Inc). Elemental analyses were carried out at the Microanalytical Centre of Cairo University, Giza, Egypt and recorded on Elementar-VarioEL (ELTRA GmbH, Haan, Germany) automatic analyzer. Compounds 1b and 16a-c were prepared by following the reported procedures in the literature [19-22]. The in vitro antimicrobial screening was performed by Chemistry of Natural and Microbial Products Dept., National Research Centre, Cairo-12622, Cairo, Egypt.
Synthesis of 9-nitro-3-phenyl-2,3,3a,4,5,6- hexahydro-2H-benzo[6,7]cyclohepteno[2,1-c]- pyrazole 3
To a mixture of compound 2b (0.29 g, 1 mmol) and hydrazine hydrate (0.4 mL, 12 mmol, 99%) in absolute ethanol (10 mL), few drops of triethylamine were added. The reaction mixture was refluxed for 4 h. After cooling, the reaction mixture was poured onto ice-water. The obtained solid was filtered off,washed with cold water, dried and crystallized from ethanol to afford the title compound 3 as gray crystals. Yield (0.25 g, 81%); mp 135-138°C; IR (KBr, cm-1) : v 3218 (NH), 1620 (C=N). 1H NMR (DMSO-d6): δ 1.68-1.79 (m, 3H, CH2 + Ha) 1.98-2.46 (m, 4H, 2CH2), 3.26 (d, 1H, Hb), 7.11-8.46 (m, 8H, Ar-H), 10.55 (br.s, 1H, NH, D2O-exchangable). 13C NMR (DMSO-d6): δ 24.15, 32.90, 113.07, 121.11, 122.87, 127.50, 127.94, 128.33, 129.45, 129.8, 131.1,135.65, 142.71, 145.31, 147.88. MS m/z (%): 307 [M+, 20], 230 (35), 115 (100), 77 (80). Anal. Calcd. For C18H17N3O2 (307.33): C, 70.34; H, 5.58; N, 13.67. Found: C, 70.56, H, 5.72, N, 13.44.
Synthesis of 2-acetyl-9-nitro-3-phenyl-2,3,3a,4,5,6- hexahydro-2H-benzo[6,7] cyclohepteno-[2,1-c] pyrazole 4
A mixture of compound 2b (0.29 g, 1 mmol) and hydrazine hydrate (0.4 mL, 12 mmol, 99%) in glacial acetic acid (10 ml) was refluxed for 3 h. After cooling, the formed precipitate was collected by filtration, dried and crystallized from ethanol to give the title compound 4 as orange crystals. Yield (0.32 g, 91%); mp 150-153°C; IR (KBr, cm-1): v 1725 (C=O), 1643 (C=N). 1H NMR (DMSO-d6): δ 1.58 (s, 3H, CH3), 1.73-1.85 (m, 3H, CH2+ Ha), 1.97-2.36 (m, 4H, 2CH2), 3.42 (d, 1H, Hb), 7.30-8.36 (m, 8H, Ar-H); MS m/z (%): 349 [M+, 15], 306 (35), 228 (40), 187 (50), 77 (70). Anal. Calcd. For C20H19N3O3 (349.14): C, 68.75; H, 5.48; N, 12.03. Found: C, 68.31; H, 5.27; N, 12.30.
Synthesis of 9-substituted 3-phenyl-3,4,5,6- tetrahydro-2H-benzo[6,7]cyclohepteno[4,3-c]- isoxazole 6a,b
To a solution of 2a,b (1 mmol) and hydroxylamine hydrochloride (0.07 g, 1 mmol) in absolute ethanol (10 mL), potassium carbonate anhydrous (0.14 g, 1 mmol) was added. The resulting mixture was heated under reflux temperature for 5-8 h and allowed to cool then diluted with water (30 mL). The solid products that formed were collected by filtration, washed with water, dried and crystallized from the proper solvents to afford the corresponding isoxazole derivatives 6a,b, respectively. 3-Phenyl-3,4,5,6-tetrahydro-2Hbenzo[ 6,7]cyclohepteno[4,3-c]isoxazole 6a. Yield (0.22 g, 84%); mp 110-112°C (EtOH/dioxin, pale yellow crystals); IR (KBr, cm-1): v 2915-2850 (3CH2).1H NMR (DMSO-d6): δ 1.79-3.21 (m, 6H, 3CH2), 6.84 -7.63 (m, 8H, Ar-H). 13C NMR (DMSO-d6): δ25.33, 31.29, 38.12,101.05, 126.5, 127.60, 127.90, 128.83, 129.01, 129.75, 131.21, 135.13, 135.67, 138.28, 155.35, 166.35. MS m/z (%): 261 [M+, 75], 188 (100), 142 (61), 77 (85). Anal. Calcd. For C18H15NO (261.32): C, 82.73; H, 5.79; N, 5.36 Found: C, 82.25; H, 5.62; N, 5.37.
9-Nitro-3-phenyl-3,4,5,6-tetrahydro-2H-benzo[6,7] cyclohepteno [4,3-c] isoxazole 6b. Yield (0.28 g, 91%); mp: 120-122°C (Dioxan, gray crystals); IR (KBr, cm-1): v 2935-2858 (3CH2). 1H NMR (DMSO-d6): δ 2.4 4-3.35 (m, 6H, 3CH2), 6.79-8.34 (m, 8H, Ar-H). MS m/z (%): 306 [M+, 46], 259 (15), 128 (51), 117 (61), 104 (36), 91 (29), 80 (100), 64 77). Anal. Calcd. For C18H14N2O3 (306.32): C, 70.58; H, 4.61; N, 9.15. Found: C, 70.37; H, 4.48; N, 9.42.
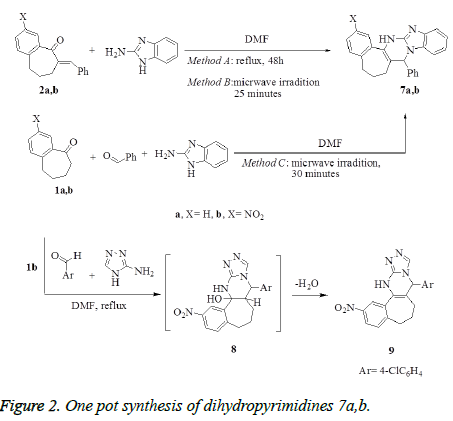
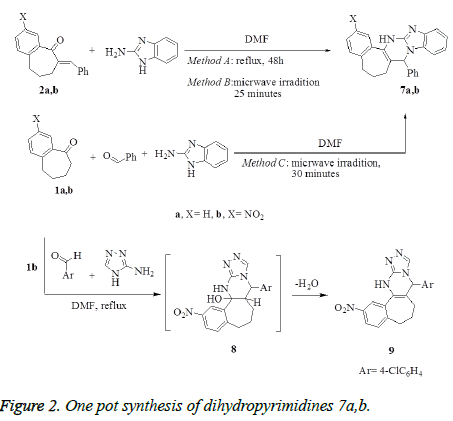
Synthesis of benzo[d]imidazo[1,2-a]pyrimidines 7a,b
Method A: A mixture of compounds 2a,b (1 mmol) and 2- aminobezimidiazole (0.13 g, 1 mmol) in anhydrous dimethylformamide (10 mL) was refluxed for 24 h. After cooling, the reaction mixture was diluted with water (30 mL), the obtained solid product was collected by filtration, washed with water, dried and crystallized from the proper solvents to afford the corresponding pentacyclic dihydropyrimidine derivatives 7a,b, respectively.
Method B: To a solution of benzylidene benzosuberones 2a,b (1 mmol) in anhydrous dimethyl-formamide (10 mL), 2- aminobenzimidazole (0.13 g, 1 mmol) was added. The reaction mixture was subjected to microwave irradiation for 25 minutes and temperature 120°C, then left to cool and diluted with cold water. The formed solid product was collected by filtration, washed with EtOH, dried, and crystallized from the proper solvents to afford pentacyclic dihydropyrimidine derivatives 7a,b.
Method C: A mixture of benzaldehyde (0.11g, 1 mmol), appropriate benzosuberones 1a,b (1 mmol) and 2- aminobenzimidazole (0.13 g, 1 mmol) in anhydrous dimethylformamide (10 mL), was heated under temperature 120°C using microwave irradiation for 30 minutes, then left to cool and diluted with cold water. The solid product that formed was filtrated off, washed with ethanol, dried, and finally recrystallized from the proper solvents to afford pentacyclic dihydropyrimidine derivatives 7a,b. The products that prepared by methods B and C were identical in all respects (mp, TLC and spectra) with that prepared from method A.
8-Phenyl-1,9,10,11- tetrahydrobenzo [6`,7`] cyclohepteno [d]-1H-benzo [d]imidazo-[1,2-a]-pyrimidine 7a. Yield: (Method A: 0.25g, 68%; Method B: 0.28g, 75%; Method C: 0.4g, 90%); mp: 175-178°C white crystals (EtOH/dioxan). IR (KBr, cm-1): v 3431(NH), 1625 (C=N). 1H NMR (DMSO-d6): δ 1.61-2.25 (m, 6H, 3CH2), 5.63 (s, 1H, CH), 6.58 (s, 1H, NHD2O- exchangeable), 7.02- 8.61(m, 12H, Ar-H). MS m/z (%): 363 [M+, 100], 172 (35), 115 (17), 91 (10), 77 (25). Anal. Calcd. For C25H21N3 (363.45): C, 82.61; H, 5.82; N, 11.56. Found: C, 82.14; H, 5.65 N, 11.90.
8-Phenyl-14-nitro-1,9,10,11- tetrahydrobenzo [6`,7`] cyclohepteno [d]-1H-benzo [d]imidazo-[1,2-a]pyrimidine 7b. Yield: (Method A: 0.17 g, 65%; Method B: 0.21 g, 77%; Method C: 0.3 g, 90%); mp: 205-207°C yellow crystals (EtOH/ dioxan). IR (KBr, cm-1): v 3433 (NH), 1603 (C=N). 1H NMR (DMSO-d6): δ 1.99-2.36 (m, 6H, 3CH2), 5.59 (s, 1H, CHpyrimidine), 7.41-8.57 (m, 12H, Ar-H), 8.57 (s, 1H, NH-D2Oexchangeable). 13C NMR (DMSO-d6): δ24.70, 26.12, 39.80, 56.44, 108.50, 115.20, 121.46, 123.67, 125.43, 126.01, 126.75, 127.12, 127.74, 129.36, 134.21, 136.21, 137.07, 138.90, 139.79, 141.11, 143.62, 154.66. MS m/z (%): 408 [M+, 15], 292 (28), 172 (10), 128 (14), 115 (20), 91 (18), 80 (98), 64 (100). Anal. Calcd. For C25H20N4O2 (408.45): C, 73.51; H, 4.94; N, 13.72. Found C, 73.31; H, 4.80; N, 14.13.
Synthesis of 6-(4-chlorophenyl)-11- nitro-6,7,8,9,10,11,13-hexahydrobenzo[6`, 7`]cyclohepta-[d]-[1,2,4]-triazolo[4,3-a]pyrimidine 9
To a stirred solution of nitrobenzosuberone 1b (1 mmol), 4- chlorobenzaldehyde (0.14 g, 1 mmol) in dimethylformamide (10 mL), 2-aminotriazole (0.085 g, 1 mmol) was added. The reaction mixture was refluxed for 9 h, allowed to cool and diluted with water (50 mL). The solid product formed was collected by filtration, washed with water. The crude product was purified by recrystallization from dioxan to give compound 9 as white crystals. Yield: 84%; mp: >300°C (Dioxan); IR (KBr, cm-1): v 3331 (NH), 1630 (C=N). 1H NMR (DMSO-d6): δ 1.71-2.65 (m, 6H, 3CH2), 6.01 (s, 1H, CHpyrimidine), 7.20-8.12 (m, 7H, Ar-H), 8.24 (s, 1H, CHtriazole), 8.58 (s, 1H, NH-D2O- exchangeable). MS m/z (%): 393 [M+, 100], 347 (35), 237(20), 111 (50), 77 (25). Anal. Calcd. For C20H16ClN5O2 (393.83): C, 60.99; H, 4.09; N, 17.78. Found: C, 60.75; H, 3.97; N, 17.92.
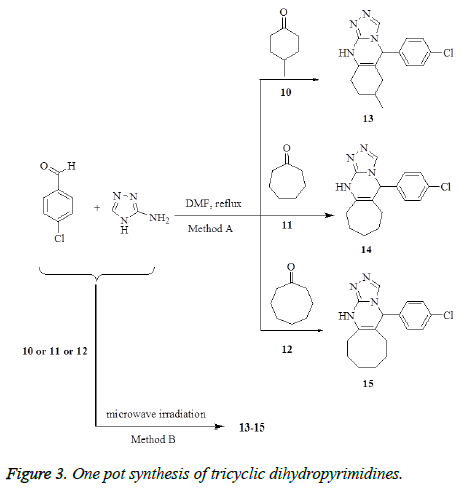
Synthesis of triazolo[3,4-b]quinazoline derivatives 13-15
Method A: To a stirred solution of the appropriate cycloalkanones 10, 11 or 12 (1 mmol) and 4- chlorobenzaldehyde (0.14 g, 1 mmol) in DMF (10 mL), 2- aminotriazole (0.085 g, 1 mmol) was added. The reaction mixtures were refluxed for 8-12 h (monitored by TLC), allowed to cool and 50 mL water was then added. The solid products that formed were collected by filtration, washed with water. The crude products were purified by recrystallization from dioxan to give 13, 14 and 15, respectively.
Method B: A mixture of 4-chlorobenzaldehyde (0.14 g, 1 mmol), the appropriate cycloalkanone 10, 11 or 12 (1 mmol) and 2-aminotriazole (0.085 g, 1 mmol) in DMF (10 mL), was heated at 120°C using microwave irradiation for 30 minutes then left to cool and diluted with cold water. The solid product that formed was filtrated off, washed with ethanol, dried, and finally recrystallized from dioxan to afford 13, 14 and 15, respectively. The products that prepared by method B were identical in all respects (mp, TLC and spectra) with that prepared from method A.
5-(4-Chlorophenyl)-7-methyl-5,6,7,8,9,10-hexahydro- [1,2,4]triazolo[3,4-b]-quinazoline 13. Yield: (Method A: 0.22 g, 75%; Method B: 0.27 g, 89%); mp: 270-272°C as white crystals. IR (KBr, cm-1): v 3341 (NH), 1635 (C=N). 1H NMR (DMSO-d6): δ 1.29 (d, 3H, CH3), 1.81-2.45 (m, 6H, 3CH2), 3.30 (m, 1H, CH), 5.71 (s, 1H, CH), 7.30-7.60 (m, 4H, Ar-H), 8.30 (s, 1H, CH), 8.32 (s, 1H, NH-D2O- exchangeable). MS m/z (%): 300 [M+, 100], 300 [M+] (100), 189 (50), 120 (20), 113 (70), 77 (30). Anal. Calcd. For C16H17ClN4 (300.79): C, 63.89; H, 5.70; N, 18.63. Found C, 62.98; H, 5.53; N, 19.18.
5-(4-Chlorophenyl)-5,6,7,8,9,10,11,13-hexahydrocyclohepta[d] [1,2,4]triazolo[4,3-a]pyrimidine 14. Yield: (Method A: 0.18 g,60%; Method B: 0.25 g, 83%); mp: >300°C as white crystals. IR (KBr, cm-1): v 3335 (NH), 1640 (C=N). 1H NMR (DMSOd6): δ 1.30-2.13 (m, 10H, 5CH2), 5.57 (s, 1H, CH-pyrimidine), 7.10-7.4 (m, 4H, Ar-H), 8.15 (s, 1H, NH-D2O- exchangeable), 8.34 (s, 1H, CH-triazole). MS m/z (%): 301 [M++ 1, 100], 189 (60), 111 (70), 70 (30), 55(20). Anal. Calcd. For C16H17ClN4 (300.79): C, 63.89; H, 5.70; N, 18.63. Found C, 63.68; H, 5.53; N, 18.78.
5-(4-Chlorophenyl)-5,7,8,9,10,11,12,14- octaahydrocycloocta[d][1,2,4]triazolo [4,3-a]-pyrimidine 15. Yield: (Method A: 0.20 g, 63%; Method B: 0.24g, 76%); mp: > 300°C as white crystals. IR (KBr, cm-1): v 3330 (NH), 1633 (C=N). 1H NMR (DMSO-d6): δ 1.30-2.13 (m, 10H, 5CH2), 5.57 (s, 1H, CH-pyrimidine), 7.14-7.43 (m, 4H, Ar-H), 8.34 (s, 1H, CH-triazole), 8.55 (s, 1H, NH, D2O-exchangeable). MS m/z (%): 314 [M+, 100], 203 (40), 111 (70), 84(35), 77 (60). Anal. Calcd. For C17H19ClN4 (314.81): C, 64.86; H, 6.08; N, 17.80. Found C, 64.98; H, 6.13; N, 17.72.
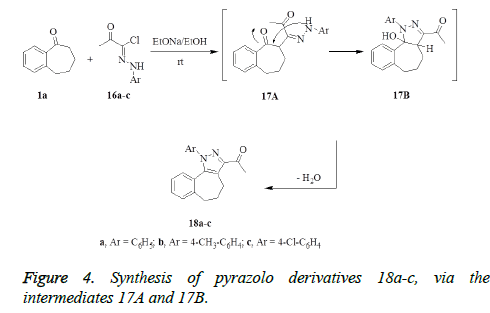
Synthesis of 3-acetyl-1-(substituted phenyl)-4,5,6- trihydrobenzo[6,7]cyclohepteno[1,2-c]-pyrazol 18a-c
To a stirred sodium ethoxide solution [prepared from sodium metal (0.11 g) and absolute ethanol (20 mL)], benzosuberone 1a (0.32 g, 2 mmol) was added. The mixture was stirred for 30 min, and then appropriate hydrazonoyl halides 16a-c (2 mmol) was added. The reaction mixture was stirred at room temperature for 24 h. The formed solid was collected by filtration, washed with water, dried and crystallized from ethanol to afford the corresponding pyrazole derivatives 18a-c, respectively.
3-Acetyl-1-phenyl-4,5,6-trihydrobenzo[6,7]cyclohepteno[1,2- c]pyrazol 18a. Yield: (85%); mp: 240-242°C as yellow powder. IR (KBr, cm-1): v 1670 (C=O). 1H NMR (DMSO-d6): δ 1.75-2.30 (m, 6H, 3CH2), 3.43 (s, 3H, CH3), 6.96-7.30 (m, 9H, Ar-H). 13C NMR (DMSO-d6): δ 23.13, 26.60, 32.0, 38.20, 116.44, 120.20, 126.50, 128.90, 135.11, 139.67, 141.28, 150.13, 196.10, 195.25. MS m/z (%):302 [M+, 45], 259 (25), 183 (30), 77 (100). Anal. Calcd. For C20H18N2O (302.14): C, 79.44; H, 6.00; N, 9.26. Found: C, 79.06; H, 5.82; N, 9.53.
3-Acetyl-1-tolyl-4,5,6-trihydrobenzo[6,7]cyclohepteno[1,2- c]pyrazol 18b. Yield: (70%); mp: 260-262°C as golden powder. IR (KBr, cm-1): v 1670 (C=O). 1H NMR (DMSO-d6): δ 1.75-2.30 (m, 6H, 3CH2), 2.55 (s, 3H, CH3), 3.23 (s, 3H, CH3), 6.96-7.30 (m, 8H, Ar-H).MS m/z (%):316 [M+, 70], 273(25), 183 (40), 91 (100). Anal. Calcd. For C21H20N2O (316.40): C, 79.72; H, 6.37; N, 8.85. Found C, 79.33; H, 6.18; N, 9.11.
3-Acetyl-1-(4-chlorophenyl)-4,5,6- trihydrobenzo[6,7]cyclohepteno[1,2-c]pyrazol 18c. Yield: (80%); mp: 275-277°C as yellow powder. IR (KBr, cm-1): v 1670 (C=O). 1H NMR (DMSO-d6): δ 1.75-2.20 (m, 6H, 3CH2), 3.13 (s, 3H, CH3), 6.86-7.20 (m, 8H, Ar-H). MS m/z (%): 336 [M+, 45], 293 (45), 183 (15), 111 (100). Anal. Calcd. For C20H17ClN2O (336.81): C, 71.32; H, 5.09; N, 8.32. Found C, 71.19; H, 4.94; N, 8.56.
Antimicrobial activity
Strains used: The antimicrobial activity of the synthesized compounds was screened against common pathogenic and food spoilage microorganisms were selected for their relevance in bakery products and other food: gram-positive (Staphylococcus aureus ATCC 29213 and Bacillus subtilits ATCC 6633) and gram-negative (Escherichia coli ATCC 2592 and Pseudomonas aeruginosa ATCC 27953) bacteria, yeast (Candida albicans NRRL Y-477) and filamentous fungi (Aspergillus niger ATCC 1015 and Aspergillus flavus ATCC 16883).
Bioassay: Chemical compounds were individually tested against a panel of gram positive and gram negative bacterial pathogens, yeast and fungi. Antimicrobial tests were carried out by the agar well diffusion method [23] using 100 μL of suspension containing 1 × 108 CFU/mL of pathological tested bacteria, 1 × 106 CFU/ mL of yeast and 1 × 104 spore/ml of fungi spread on nutrient agar (NA), plates each containing 25 mL of the respective, Sabourand Dextrose Agar (SDA), and Potato Dextrose Agar (PDA) media, respectively, to reach a final concentration of 0.015 (O600). After the media had cooled and solidified, wells (10 mm in diameter) were made in the solidified agar and loaded with 100 μL of tested compound solution prepared by dissolving 10 mg of the chemical compound in one ml of dimethyl sulfoxide (DMSO).
The inculcated plates were then incubated for 24 h at 37, 26 and 28°C for bacteria, yeast and for 48 h for fungi, respectively. Negative controls were prepared using DMSO employed for dissolving the tested compound. Vebromycine (10 mg/ml) and Ketoconazole (10 mg/ml) were used as standard for antibacterial and antifungal activity respectively. After incubation time, antimicrobial activity was evaluated by measuring the zone of inhibition against the test organisms and compared with that of the standard. The observed zone of inhibition is presented in Table 1. Antimicrobial activities were expressed as inhibition diameter zones in millimeters (mm). The experiment was carried out in triplicate and the average zone of inhibition was calculated.
Minimal inhibitory concentration (MIC) measurement
The bacteriostatic activity of four active compounds 7b, 9, 13 and 14 was then evaluated using the two fold serial dilution technique (Table 2) [24]. Two fold serial dilutions of the tested compounds solutions were prepared using the proper nutrient broth. The final concentration of the solutions were 1000, 500, 250, and 125 μg/mL. The tubes were then inoculated with the test organisms, grown in their suitable broth at 37°C for 24 hours for tested microorganisms (1 × 108 CFU/mL for bacteria, 1 × 106 CFU/mL of yeast and 1 × 104 spore/ml for fungi), each 5 ml received 0.1 ml of the above inoculum and incubated at 37°C for 24 hours. The lowest concentration showing no growth was taken as the Minimum Inhibitory Concentration (MIC).
Results and Discussion
Chemistry
6- Benzylidene-3- nitro-6,7,8,9- tetrahydrobenzo [6,7] cyclohepten-5- one 2 was synthesized as starting material by reacting of 3-nitro-6,7,8,9-tetrahydro-5H-benzocyclohepten-5- one 1a,b with benzaldehyde according to the reported procedures (Figure 1) [21,22]. Condensation of 2b with hydrazine hydrate in ethanol containing a catalytic amount of triethylamine gave 9-nitro-3-phenyl-2,3,3a,4,5,6- hexahydrobenzo[6,7]cyclohepteno[2,1-c]pyrazole 3 (Figure 1). Meanwhile, reaction of 2b with hydrazine hydrate in glacial acetic acid afforded 2-acetyl-9-nitro-3-phenyl-2,3,3a,4,5,6- hexahydro-2H-benzo[3,4]cyclohepteno[2,1-c]pyrazole 4. The benzosuberone derivatives 2a and 2b were reacted with hydroxylamine hydrochloride, afforded the corresponding 3- phenyl-3,4,5,6-tetrahydro-2H-benzo[6,7]cyclohepteno[4,3- c]isoxazole 6a as pale yellow crystals and 9-nitro-3- phenyl-3,4,5,6-tetrahydro-2H-benzo[6,7]cyclohepteno[4,3- c]isoxazol 6b as gray crystals in 84% and 91% yield, respectively (Figure 1). This reaction of 6-benzylidene-6,7,8,9- tetrahydro-3-nitrobenzo[7]annulen-5-ones 2a and 2b with hydroxylamine proceeds by initial condensation, followed by cyclization of intermediate 5a,b via nucleophilic addition to give 6a,b as presented in Figure 1.
Treatment of benzylidene derivatives 2a,b with 2- aminobenzimidazole in refluxing DMF afforded pentacyclic dihydropyrimidines (DHPs) 7a,b, respectively (Method A), which were obtained also by using microwave irradiation (Method B). One pot synthesis, as alternative method, was applied to prepare the dihydropyrimidines 7a,b by heating the mixture of 1a,b with benzaldehyde and 2-aminobenzimidazole in DMF using microwave irradiation (Method C) (Figure 2). The usage of microwave in the synthesis of 7a,b (methods B and C) has proved to be time saving and surprisingly increasing the yield. Treatment of 1b with 4- chlorobenzaldehyde and 2-aminotriazole in one pot reaction in DMF under reflux afforded the cyclized tetracyclic dihydropyrimidine derivative 9 via the intermediate 8 (Figure 2).
We have extended our synthetic strategy towards polycyclic dihydropyrimidines via three components one pot synthesis. To obtain these targets we have used monocyclic ketone instead of benzosuberone with 4-chlorobenzaldehyde and 2- aminotriazole. Thus, the reaction of 4-chlorobenzaldehyde with 2-aminotriazole and cycloalkanone derivatives 10-12, in refluxing DMF for 8-12 h, afforded the corresponding tricyclic dihydropyrimidines 13-15, in 75%, 60% and 63% yield, respectively (Method A, Figure 3). In light of the aforementioned data, we have used the microwave irradiation as a tool in performing this one pot reaction. The same one pot reactions were performed in DMF under microwave irradiation at 120°C for 30 minutes to give the corresponding tricyclic dihydropyrimidines 13-15, in 84%, 89%, 83% and 76% yield respectively (Method B, Figure 3).
The reaction of benzosuberone 1a with (1-(2-(4-substituted phenyl)hydrazono)-1-chloropropan-2-ones 16a-c in the presence of sodium ethoxide in absolute ethanol at room temperature with stirring afforded the corresponding pyrazolo derivatives 18a-c, via the intermediates 17A and 17B (Figure 4).
Antimicrobial evaluation
The in vitro antimicrobial activity of the synthesized products was performed against the tested pathogens represented by bacteria, yeast and fungi in comparison with the standard antibiotics used vebromycine and ketoconazole (Table 1). Compounds 7b, 9, 13, 14 and 15 have shown a strong inhibitory effect against gram-positive bacteria (S. aureus). Meanwhile, compounds 4, 13, 14 and 16 were shown to be active against B. subtilits. In case of gram-negative bacteria, all the tested products showed a remarkable activity against E. coli. The tested products have shown a strong to moderate effect against most of. Products 3, 4, 9, 13, 14, 15 and 18a have shown a strong activity against P. aeruginosa.
| Compound no | Bacteria | Yeast | Fungi | ||||
|---|---|---|---|---|---|---|---|
| Gram +ve | Gram -ve | ||||||
| S. aureus | B. subtilis | E. coli | P. aeroginosa | C. Albicans | A. Niger | A. flavus | |
| 3 | 12 | 18 | 18 | 18 | 18 | 15 | 21 |
| 4 | 20 | 22 | 20 | 20 | 30 | 25 | 28 |
| 6b | 15 | 19 | 25 | N.A. | 30 | 28 | 25 |
| 7b | 25 | 15 | 25 | N.A. | 25 | 20 | 27 |
| 9 | 25 | N.A. | 25 | 15 | 25 | 20 | 26 |
| 13 | 30 | 26 | 15 | 25 | 15 | N.A | 17 |
| 14 | 30 | 25 | 15 | 24 | N.A. | N.A. | N.A. |
| 15 | 30 | 28 | N.A. | 20 | N.A. | N.A. | N.A. |
| 18a | N.A. | N.A. | 20 | 15 | 22 | 20 | 26 |
| 18b | 20 | N.A. | 15 | N.A. | 19 | N.A. | 15 |
| 18c | N.A. | N.A. | 15 | N.A. | 17 | N.A. | 15 |
| Vebromycine | 20 | 22 | 25 | 24 | N.A. | N.A. | N.A. |
| Ketoconazole | N.A. | N.A. | N.A. | N.A. | N.A. | 22 | 23 |
The experiment was carried out in triplicate and the average zone of inhibition was calculated
Table 1. Antimicrobial activity expressed as inhibition diameter zones in millimeters (mm) of chemical compounds against the pathological strains based on well diffusion assay.
The antifungal activities of compounds 4, 6b, 7b and 9 showed to be strong against C. albicans. Whereas, compounds 4, 6b, 7b, 9 and 18a have a higher effect than the standard (Ketoconazole), in case of filamentous fungi (A. niger and A. flavus). Also, the broad spectrum effect of compounds 3 and 4 against all tested pathogens: gram-positive bacteria, gramnegative bacteria, and fungi, indicated that these compounds can be used in the treatment of the tested pathogens. The Minimum Inhibitory Concentration (MIC) of the tested products 7b, 9, 13 and 14 is presented in Table 2. The MIC of the compounds 13 and 14 was 125 μg/mL against S. aureus and was 250 μg/mL against B. sutbtilis. MIC was 250 μg/mL for the tested compounds 7b and 9 for their antibacterial (S. aureus and E. coli) and antifungal (C. Albicans and A. flavus) activities. Finally, the structure-activity relationships (SAR) of these synthesized compounds (polycyclic derivatives) can be attributed to their ability to inhibit the cell growth by inhibiting the protein synthesis [25,26].
| Compound no. | Gram +ve | Gram -ve | Yeast | Fungus | |||
|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. aeroginosa | C. Albicans | A. Niger | A. flavus | |
| 7b | 250 | 1000 | 250 | - | 250 | 500 | 250 |
| 9 | 250 | - | 250 | 1000 | 250 | 500 | 250 |
| 13 | 125 | 250 | 1000 | 250 | 1000 | - | 500 |
| 14 | 125 | 250 | 1000 | 250 | -. | - | - |
| Vebromycine | 125 | 125 | 125 | 125 | - | - | - |
| Ketoconazole | - | - | - | - | 125 | 125 | - |
The experiment was carried out in triplicate and the average zone of inhibition was calculated
Table 2. Minimum inhibitory concentration (MIC, μg/ml) against the pathological strains based on two fold serial dilution technique.
Acknowledgement
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
References
- Antimicrobial Resistance Global Report on Surveillance, World Health Organization, Geneva, Switzerland, 2014.
- Nikaido H. Multidrug resistance in bacteria. Annual Rev Biochemistry 2009; 78: 119-146.
- Hamada NMM, Sharshira EM. Synthesis and antimicrobial evaluation of some heterocyclic chalcone derivatives. Molecules 2011; 16: 2304-2312.
- Azarifar D, Shaebanzadeh M. Synthesis and characterization of new 3,5-dinaphthyl substituted2-pyrazolines and study of their antimicrobial activity. Molecules 2002; 7: 885-895.
- Korgaokar SS, Patil PH, Shah MJ, Parekh HH. Studies on pyrazolines: Preparation and antimicrobial activity of 3-(3’-(p-chlorophenyesulphonamidophenyl)-5-aryl-1H-acetyl-pyrazolines. Ind J Pharm Sci1996; 58: 222-225.
- Ali MA, Siddiqui AA, Shaharyar M. Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur J Med Chem2007; 42: 268-275.
- Mui MS, Siew BN, Buss AD, Crasta SC, Kah LG, Sue KL. Synthesis of N-1 acidic functionality affording analogues with enhanced antiviral activity against HIV. Bioorg Med ChemLett2002; 12: 679-699.
- Dawood KM, Eldebss TMA, El-Zahabi HSA, Yousef MH, Metz P. Synthesis of some new pyrazole-based 1,3-thiazoles and 1,3,4-thiadiazoles as anticancer agents. Eur J Med Chem2013; 70: 740-749.
- Nassar E. Synthesis, (in vitro) Antitumor and antimicrobial activity of some pyrazoline, pyridine and pyrimidine derivatives linked to indoles moiety. J Am Sci2010; 6: 463-471.
- Amir M, Kumar H, Khan SA. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg Med ChemLett2008; 18: 918-922.
- Siddiqui AA, Rahman MA, Shaharyar M, Mishra R. Synthesis and anticonvulsant activityof some substituted 3,5-diphenyl-2-pyrazoline-1-carboxamide derivatives. J ChemSci2010; 8: 1-10.
- Prasad YR, Rao AL, Prasoona L, Murali K, Kumar PR. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2H-hydroxynaphthalen-1-yl)-1,5-di-phneyl-2-pyrazolines. Bioorg Med ChemLett2005; 15: 5030-5034.
- Bilgin AA, Palaska E, Sunal R. Studies on the synthesis and antidepressant activity of some1-thiocarbamoyl-3,5-diphenyl-2-pyrazolines. ArzneimForsch Drug Res 1993; 43: 1041-1044.
- Abd El-Salam OI, Abou El Ella DA, Ismail NSM. Synthesis, docking studies and anti-inflammatory activity of some 2-amino-5,6,7,8-tetrahydroquinoline-3-carbonitriles and related compounds.Pharmazie2009; 64: 147-155.
- Abdel Hafez NA, Abd El-Salam OI, Hammam AG. Synthesis of some F-heterocyclic compounds of potential anticancer activity using 8-fluoro-1-benzosuberon as a synthon. Egypt J Chem2006; 49: 63-71.
- Hammam AG, Abd El-Salam OI, Mohamed AM, Abdel Hafez NA. Novel fluoro substituted benzo[b]pyran with antilung cancer activity. Indian J Chem2005; 44: 1887-1893.
- Abd El-Salam OI, Khalifa NM, Said SA, Amr AE. Synthesis and antimicrobial activities of some newly 2,4,6-trisubstituted pyridine derivatives, ResChemIntermed 2014; 40: 1147-1155.
- Amr AE, Mohamed AM, Mohamed SF, Abdel Hafez NA, Hammam AG. Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives, Bioorg Med Chem2006; 14: 5481-5488.
- Abd El-Salam OI, Ali S A., Ali KA, AbdElwahab AA, Amr AE, Awad HM. Synthesis and Antimicrobial Evaluation of a New Series of Heterocyclic Systems Bearing a Benzosuberone Scaffold, Molecules 2015; 20: 20434-20447.
- Abd El-Salam OI, Al-Omar MA, Khalifa NM, Amr AE, Abdallah MM. Analgesic and anticonvulsant activities of some newly synthesized trisubstituted pyridine derivatives. Z. Naturforsch2013; 68: 264-268.
- Smith PA, Berry WL. Some exploratory syntheses of benzosuberans and tetrahydro-benzazapinones and some related diazoxides. J Org Chem1961; 26: 27-34.
- Ali MI, Hammam AG. Chemistry of Seven-Membered Heterocycles. 4. Synthesis and Reactions of 8-Aryl-6,7,10,11-tetrahydro-5H,8H-benzo[3,4]cycloheptino[2,1-d]-thiazolo[3,2-a]-pyrimidin-10-ones. J ChemEng Data 1978; 23: 91-93.
- Perez C, Pauli M, Bazevque P. An antibiotic assay by the agar well diffusion method.ActaBiolExper 1990; 15: 113-115.
- Scott AC. Laboratory Control of Antimicrobial Therapy. In: Collee J.G., Duguid J.P., Fraser A.G., Marmion B.P., Mackie and MacCartney Practical Medical Microbiology. 13th Ed, Churchill Livingstone, 1989, 2: 161-181.
- Connell SR, Tracz DM, Nierhaus KH, Taylor DE. Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrob Agents Chemother2003; 47: 3675-3681.
- Hinman MM, Rosenberg TA, Balli D, Black-Schaefer C, Chovan LE, Kalvin D, Merta PJ, Nilius AM, Pratt SD, Soni NB. Novel antibacterial class: A series of tetracyclic derivatives. J Med Chem2006; 49: 4842-4856.