- Biomedical Research (2014) Volume 25, Issue 2
Anti-inflammatory and antioxidant effects of ethyl pyruvate on colitis: In a rat model.
Nazım Güreş1, Meral Karaman2, Cengiz Tavusbay3*, Efsun Kolatan2, Aslı Çelik2, Çetin Pekçetin4,Zahide Çavdar5, Tuncay Küme6, Ensari Guneli2, Osman Yılmaz21Acibadem International Hospital, Department of General Surgery, Yesilkoy-Istanbul/TURKEY
2Department of Laboratory Animal Science, Dokuz Eylul University, Faculty of Medicine, Inciralti-Izmir/Turkey
3Katıp Celebi University, Ataturk Training and Education Hospital, Department of general surgery, Karabaglar-Izmir/Turkey
4Department of Histology and Embriology, Dokuz Eylul University, Faculty of Medicine, Inciralti-Izmir/Turkey
5Department of Molecular Medicine, Health Science Institute, Dokuz Eylül University, Faculty of Medicine, Inciralti- Izmir/Turkey
6Department of Medical Biochemistry, Dokuz Eylul University Faculty of Medicine, Inciralti-Izmir/Turkey.
- *Corresponding Author:
- Cengiz Tavusbay
Erzene Mah Sehit Taha Carim Cad. Özveri Sitesi No: 3/1
B Kat:3, Da: 5
Bornova 35050 Izmir,Turkey
Accepted January 04 2014
Abstract
In this study, we analyzed the anti-inflammatory and antioxidant effects of ethyl pyruvate (EP) in an experimental colitis model. Seventeen Wistar Albino rats were divided into three groups: The sham group (n=3), in which no colitis was induced; the EP (n=7) and normal saline (NS) (n=7) group, in which colitis was induced. In EP and NS groups, 50 mg/kg of EP or NS was administered subcutaneously for 7 days. Weight changes in the rats were observed throughout the study. All rats were sacrificed on the seventh day. The injury in the resected distal colons was scored histopathologically and tissue malonyl dialdehyde (MDA) levels were measured. The injury scores of the NS group were found to be higher than those of the Sham group, whereas the scores were lower in the EP-administered group in comparison with both Sham and NS groups (p=0.002 and p=0.003, respectively). Although tissue MDA levels in the EP group were lower than that in the NS group, the difference was not significant. Although there was an apparent weight increase in the EP group compared with the NS group, it was not found to be statistically significant. The histopathological data indicated that EP reduced inflammatory injury in rats with colitis.
Keywords
colitis, ethyl pyruvate, histopathology, injury score, inflammatory bowel disease
Introduction
Colitis, characterized by intestinal inflammation and changes in bowel functions, is an inflammatory process involving colonic mucosa. Inflammatory bowel disease (IBD), leading to colitis, is a chronic progressive disease, which involves the gastrointestinal system, and is accompanied by unexplained morbidity and mortality. IBD not only causes symptoms of decreasing quality of life, such as chronic abdominal pain, hemorrhagic diarrhea, and fever, but also increases the risk of cancer, significantly. In addition, it leads to socioeconomic problems throughout the world because of work force losses and treatment costs. The characteristics of IBD are inflammation and tissue destruction, and clinical features and morphological changes develop as a result of the activation of inflammatory cells, in particular neutrophils and lymphocytes. Although many surgical and medical treatment protocols are available, new options for treatment are still being sought out [1]. The basic goal of medical treatment is to attenuate the severity of the disease by means of suppressing the inflammatory response [2].
In recent years, some studies have been carried out to highlight the importance of the nuclear factor-κβ (NF-κβ)- mediated intracellular signal transmission in inflammation, and blocking this pathway has been shown to alleviate the inflammatory response and therefore IBD [3-6]. Ethyl pyruvate (EP), the aliphatic ester of pyruvate, is a molecule produced endogenously by the glycolytic pathway. In the literature, EP has been reported to exert anti-inflammatory and antioxidant effects by blocking the NF-κβ transmission system. In cells stimulated with various stimuli (ischemia, cytokines, growth factors, etc.), cytoplasmic NF-κβ is carried to the cell nucleus, where it binds to the DNA and thereby interacts with various genes. This factor plays a significant role in congenital and acquired immunity, inflammation, bone formation, and cancer pathogenesis [7].
In this study, the objective was to investigate the antiinflammatory and antioxidant effects of EP in an experimental colitis model induced in Wistar albino rats.
Material and Methods
Study Groups
Seventeen adult male Wistar albino rats (200-250 g), bred using conventional methods, were used in this study. The rats were caged under standard environmental conditions (room temperature [22º C ± 1º C], humidity 50-70%, photoperiod 12 hours of light, 12 hours of darkness) and supplied with standard feed and water. The study was conducted with the approval of the local board of ethics in compliance with ethical rules (Ethics Committee Approval Number: 62/2008). The groups, comprised of randomly selected experimental animals, were formed as follows:
1. Sham Group (n=3): No colitis was induced in this group, normal saline (NS) was administered rectally and no treatment protocol was applied.
2. Colitis + NS Group (n=7): In this group, after induction of colitis, a daily dose of 50 mg/kg NS was administered subcutaneously for 7-days.
3. Colitis + EP Group (n=7): After induction of colitis, a daily dose of 50 mg/kg EP was administered subcutaneously for 7-days, in this group [8].
Induction of Colitis in the animals
After 12 hours of starving, the rats were anesthetized on the day of the experiment with a combination of ketamine (35 mg/kg) and xylazine (5 mg/kg). Distal colitis was induced by rectal administration of 2 mL of 6% acetic acid [9]. After inducing colitis, weight changes in the rats were monitored on the 1st, 4th, and the 7th day. At the end of Day 7, all animals were sacrificed with a lethal dose of anesthetic, the abdomen was opened through a midline incision, and an 8 cm segment of the distal colon was resected [10].
Histopathological Analysis
A 2 cm segment of the resected distal colon was kept in 10% formaldehyde for histopathological analysis. After routine tissue follow-up, the tissues were embedded in paraffin blocks. Thin slices (4 μm sections) were stained with hematoxylin-eosin and analyzed blindly by two researchers under light microscopy. In order to evaluate the inflammation, the degree of the injury was scaled between 0-3; 0 = no inflammation, 1 = increase in the number of mucosal, submucosal, or transmural lymphocytes/neutrophils, 2 = increase in the number of mucosal lymphocytes and neutrophils; and 3 = regeneration of crypts, erosion, ulcers, and mucosa [11].
Malonyldialdehyde (MDA) Analysis
Following the homogenization and extraction of the distal colon segments, the supernatants were transferred into sample dishes for high-performance liquid chromatography (HPLC). Analyses were done using a Shimadzu-VP series HPLC system (Shimadzu Scientific Instruments Inc., Columbia, MD). The instrument is composed of an LC- 10ADVP pump, SCL 10AVP system control unit, RF- 10AXL fluorescence detector, SPD-10AVP visible/UV light detector, SIL-10ADVP autoinjector, CTO-10ASVP column oven, and FCV-10AVP gradient mixer. MDA levels of the tissue samples were calculated according to a standard calibration curve, with 1,1,3,3-tetraethoxypropane used as the standard. Tissue MDA levels were expressed in millimole MDA/mg protein (12).
Statistical Analysis
The nonparametric Mann Whitney U test was used for comparison of the groups.
Results
Weight Changes in Rats
The weight increase in the EP group on the first, fourth, and seventh days of colitis was greater when compared with that of the NS group, however there was no significant difference between the groups (p>0.05). On the other hand, the weights of the rats in the EP and NS groups (in which colitis had been induced), decreased by the end of the third day, then started to increase by the fourth day and went beyond the baseline by the seventh day.
During the first three days of colitis, diarrhea was seen in the EP and NS groups. The rats were observed to squat down in a humped position to help relieve abdominal pain. Frequent defecation and extremely watery stool were observed in the NS group.
Histopathological Findings
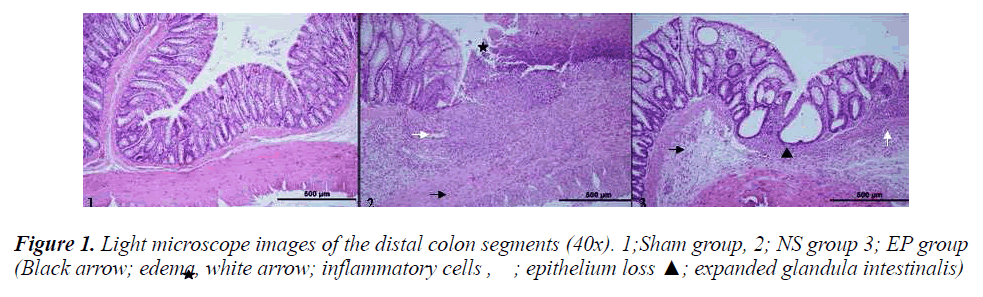
There was no macroscopic change in the distal colon segments of the sham group, whereas mucosal ulcerations were seen in the colitis-induced groups (Figure 1).
Afterwards, the specimens were evaluated microscopically, and the injury scores were graded. There was no injury in the sham group, but significant epithelium loss and ulceration, as well as an increase in the number of mucosal, submucosal, and transmural lymphocytes/- neutrophils were observed in the NS group. However, although the numbers of mucosal and submucosal lym phocytes/neutrophils were increased, less epithelium loss and ulceration were observed in the EP group.
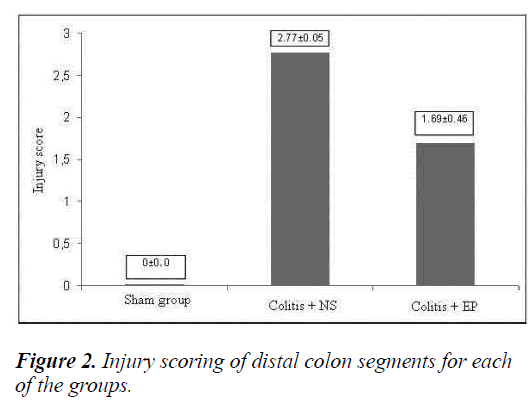
Compared with the sham group, the injury scores in the NS group were found to be higher. Whereas, the injury scores in the EP-administered group were lower when compared with both the sham and the NS groups (p=0.002 and p=0.003) (Figure 2).
Tissue MDA levels
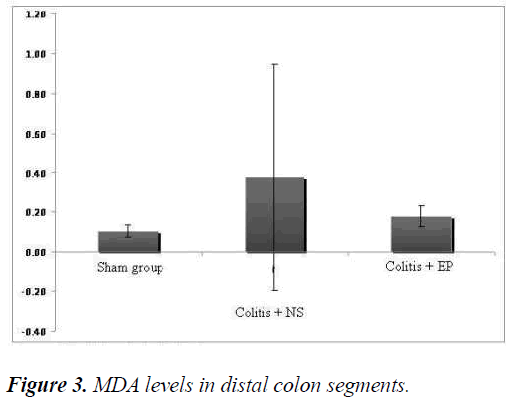
In order to evaluate oxidative stress in the distal colon segments, MDA levels were measured. The average MDA levels in the NS and EP groups were found to be higher than that in the sham group (Figure 3). Although the MDA levels in the EP group were lower than those in the NS group, the difference was not statistically significant (p=0.65'.
Discussion
Inflammatory bowel diseases such as Crohn's disease, ulcerative colitis, and indeterminate colitis are generally seen at young ages. They are among the diseases for which prevalence is gradually increasing in Western societies. Although the etiology underlying IBDs is still not precisely known, significant progress has been achieved in this field, with the description of certain pathological mechanisms and some rare environmental factors, and with the identification of the affected genes [13-15]. IBD has a course of flare-up and remission periods. Aminosalicylates, corticosteroids, immunosuppressive agents, and anti-tumor necrosis factor- α (TNF-α) drugs are medications used in the treatment of these diseases [16-18]. In recent years, together with the explanation of the role of cytokines in pathogenesis, agents like anti-adhesion molecules, anti-interleukin (IL)- 12/23, and anti-IL-6R have been included in the treatment [19]. In case of complications and lack of response to medical treatment, surgery is indicated.
In IBDs, mucosal immunity in the bowel is impaired due to the distortion of the fragile balance between proinflammatory and anti-inflammatory cytokines. For this reason, immunobiological treatments targeting TNF, leukocyte adhesion, Th1 polarization, T cell activation, and NF-κβ are gaining importance [3]. NF-κβ is a generic term for a family of transcription factors with diverse physiological functions. They are formed by dimerization of Rel proteins; Rel A (p65), c-Rel, Rel B, p50, and p52 [4]. Impairment of NF-κβ regulation leads to inflammatory and autoimmune diseases, septic shock, viral infections, and immune system disorders.
Pyruvate is an important metabolic derivative and an important scavenger of reactive oxygen radicals and hydrogen peroxide. It induces anti-inflammatory effects through NF-κβ inhibition. As pyruvate is not stable in aqueous solutions, its aliphatic ester EP has been utilized in many studies, and has also been shown to exert antiinflammatory effects [20]. Yang et al. reported that EP treatment reduced the levels of pro-inflammatory transcription factors, NF-κβ, NO synthetase, cyclooxygenase-2 (COX-2), IL-6, and TNF-α in liver, colon, and small intestine tissues in hemorrhagic shock models [21]. Furthermore, Cai et al. stated that EP, which blocks proinflammatory mediators such as TNF and highmobility group protein B1 (HMGB-1), contributed to survival in sepsis and hemorrhage models [22]. It has also been suggested that, in rats undergoing cecal ligation and puncture, EP reduces bacterial translocation, and therefore the risk of sepsis, and acts as an immunological barrier in the small intestine [23].
In light of the studies mentioned above, we used histopathological and biochemical data to investigate antiinflammatory and antioxidant effects of EP on the bowel mucosa in an experimental colitis model. According to a similar study in the literature, among IL–10-/- C57BL6 deficient rats with chronic colitis, the histological scoring of rats with systemically administered EP improved by 50%, and IL12 p40 levels in the colonic tissue were found to be significantly lower [11]. In our study, however, instead of trinitrobenzene sulfonic acid, acetic acid was used to induce colitis in rats. The colitis model was validated by both histopathological data and the observation of abdominal pain and diarrhea, which are clinical symptoms of IBD, in the rats. In the literature, the effects of EP on tissues with colitis have been determined not only through histopathological data, but also by measuring inflammatory mediators in the tissue. In our study, the antioxidant features of EP were analyzed by measuring tissue MDA levels, and colitis induction and changes observed in the colonic mucosa were scored histopathologically. Edema, inflammatory cell proliferation, epithelium loss, and transmural inflammation were more apparent in the NS group than in the EP group, and the difference between the groups was found to be statistically significant. Although the average MDA levels of the EP group were lower than that of the NS group, this difference was not statistically significant. In order to ascertain whether EP lowers the oxidative stress, additional studies should be carried out that include more subjects and investigate different parameters indicating oxidative stress.
In conclusion, this study, on the basis of histopathological data, shows that EP reduces inflammatory injury in an experimental rat colitis model. Considering the antioxidant effects reported in the literature, EP might be a useful agent for the treatment and/or attenuation of the symptoms of inflammatory bowel diseases. However, more studies are required be done in this field.
References
- Ko IK, Kim BG, Awadallah A, Mikulan J, Lin P, Letterio JJ, et al. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther 2010; 18: 1365-1372.
- Peyrin-Biroulet L, Lémann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther 2011; 33: 870-879.
- Ghosh N, Chaki R, Mandal V, Lin GD, Mandal SC. Mechanisms and efficacy of immunobiologic therapies for inflammatory bowel diseases. Int Rev Immunol 2010; 29: 4-37.
- Lawrence T, Fong C. The resolution of inflammation: anti-inflammatory roles for NF-kappaB. Int J Biochem Cell Biol 2010; 42: 519-523.
- Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease.Gut 1998; 42: 477-484.
- Andresen L, Jørgensen VL, Perner A, Hansen A, Eugen-Olsen J, Rask-Madsen J. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 2005; 54: 503-9.
- Inoue J, Gohda J, Akiyama T, Semba K. NF-kappaB activation in development and progression of cancer. Cancer Sci 2007; 98: 268-274.
- Uchiyama T, Delude RL, Fink MP. Dose-dependent effects of ethyl pyruvate in mice subjected to mesenteric ischemia and reperfusion. Intensive Care Med 2003; 29: 2050-2058.
- Aslan A, Temiz M, Atik E, Polat G, Sahinler N, Besirov E, et al. Effectiveness of mesalamine and propolis in experimental colitis. Adv Ther 2007; 24: 1085-1097.
- Kasimay O, Güzel E, Gemici A, Abdyli A. Colitis- induced oxidative damage of the colon and skeletalmuscle is ameliorated by regular exercise in rats: the anxiolytic role of exercise. Exp Physiol 2006; 91 : 897- 906.
- Davé SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D, et al. Ethyl pyruvate decreases HMGB1 release andameliorates murine colitis. J Leukoc Biol 2009; 86: 633-643.
- Lykkesfeldt J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: Comparison with ultraviolet-visible spectroscopy. Clin Chem 2000; 47:1725-1727.
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007; 369: 1641-1657.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence and environmen- tal influences. Gastroenterology 2004; 126: 1504-1517.
- Zhou N, Chen WX, Chen SH, Xu CF, Li YM. Inflammatory bowel disease unclassified. J Zhejiang Univ Sci B 2011; 12: 280-286.
- Plevy SE, Targan SR. Future therapeutic approaches for inflammatory bowel diseases. Gastroenterology 2011; 140: 1838-1846.
- Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology 2011; 140: 1827-1837.
- Villablanca EJ, Cassani B, von Andrian UH, Mora JR. Blocking lymphocytelocalization to the gastrointestinal mucosa as a therapeutic strategy forinflammatory bowel diseases. Gastroenterology 2011; 140: 1776-1784.
- Guidi L, Marzo M, Felice C, Mocci G, Sparano L, Pugliese D, et al. New biological agents for the treatment of the "high risk" IBD patients. Eur Rev Med Pharmacol Sci 2010; 14 : 342-346.
- Kao KK, Fink MP. The biochemical basis for the anti- inflammatory andcytoprotective actions of ethyl pyruvate and related compounds. Biochem Pharmacol 2010; 80: 151-159.
- Yang R, Gallo DJ, Baust JJ, Uchiyama T, Watkins SK, Delude RL, et al. Ethyl pyruvate modulates inflamema- tory gene expression in mice subjected to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 2002; 283: 212-221.
- Cai B, Deitch EA, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage. MediatorsInflamm 2010; 2010: 642462.
- Zhang Y, Li M, Meng M, Qin C. Effect of ethyl pyruvate on physical and immuno logical barriers of the small intestine in a rat model of sepsis. J Trauma 2009; 66: 1355-1364.