Research Article - Biomedical Research (2017) Volume 28, Issue 2
Anti-inflammatory and analgesic activities of some novel carboxamides derived from 2-phenyl quinoline candidates
Nagy M Khalifa1,2*, Mohamed A Al-Omar1, Ahmed A Abd El-Galil3, Mohammed Abd El-Reheem41Department of Pharmaceutical Chemistry, Drug Exploration & Development Chair (DEDC), College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
2Department of Therapeutical Chemistry, Pharmaceutical and Drug Industries Division, National Research Centre, Dokki, Cairo, Egypt
3Research Centre, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
4Department of Biochemistry, Faculty of Agriculture, Ain Shams University, Cairo, Egypt
- *Corresponding Author:
- Nagy M Khalifa
Department of Pharmaceutical Chemistry
Drug Exploration & Development Chair
College of Pharmacy
King Saud University, Saudi Arabia
Accepted on July 04, 2016
Abstract
Quinolines and its derivatives represent a broad class of compounds, which have received considerable attention due to their wide range of pharmacological properties such as, anti-inflammatory, COX inhibitor, anticancer and analgesic. Starting from 2-phenylquinoline-4-carbohydrazide, several new 2- phenylquinoline-4-carboxamide derivatives were synthesized, characterized and screened for their analgesic and anti-inflammatory activity using peripheral analgesic activity (writhing test) and carrageenan-induced paw edema test in rats. Among these derivatives, compound 5 linked nucleoside analogue showed significant anti-inflammatory activity as that of diclofenac sodium (reference drug) in animal models of inflammation.
Keywords
2-Phenyl quinoline derivatives, Carboxamides, Analgesic and anti-inflammatory activity.
Introduction
The search for new drugs which treat both infectious and inflammatory states without side effects remains a major challenge in biomedical studies. The advanced studies are enriched with progressive findings about the preparation and medicinal properties of heterocycles bearing quinoline moiety are reported to display a broad spectrum of pharmacological effects such as anti-inflammatory [1-3], analgesic [4], antimalarial [5], antimicrobial [6-9], anticancer [10], antialzheimer's [11], antioxidant [12] and antidepressant [13]. In addition, introduction of carboxamides into a skeleton of practical importance is of high interest [14,15]. In light of the aforementioned findings and our interest in the synthesis of novel heterocycles of biological importance [16-18], we have synthesized some new quinoline derivatives incorporated carboxamide linkage to evaluate their anticipated antiinflammatory activities.
Material and Methods
Melting points were measured using open capillary tubes with Griffin apparatus and are uncorrected. Elemental microanalyses were found within the acceptable limits of the calculated values. IR spectra (ν in cm-1) using KBr discs were recorded on Schimadzu 435 IR Spectrophotometer. NMR spectra (DMSO-d6) δ, ppm) were run on a Varian Gemini 500 MHz and Brucker 500 MHz Spectrophotometer with Tetramethylsilane as internal standard. Chemical shift values (δ) are given using parts per million scales (ppm). Mass Spectra (EI, 70 eV) were recorded on Hewlett Packard 5988 spectrometer. Thin layer chromatography was performed on Merck silica gel 60 F254 aluminum sheets; compound spots were visualized in UV light at 365 and 254 nm.
Synthesis of imide and bis-imide derivatives (2, 3 and 4)
General procedure: A stirred solution of hydrazide 1 (10 mmol) and acid anhydride derivatives, namely naphthalene-1,8-dicarboxylic acid anhydride, 1,2,4,5- benzenetetracarboxylic dianhydride or 1,4,5,8- naphthylenetracarboxylic dianhydride (10 mmol) in acetic acid (15 ml) was heated for 3-5 h. The reaction mixture was concentrated under reduced pressure, cooled, and the separated solid was collected by filtration, dried, and crystallized from (AcOH/ether) to afford the title imide or bis-imide derivatives 2, 3 and 4, respectively [19].
N-2-(1,3-Dioxo-3a,6-dihydro-1Hbenzo[ de]isoquinolin-2(3H)-yl)-2-phenylquinolin-4- carboxamide (2).
Yield 62%, m.p. 201-203°C; IR (KBr, cm-1): ν 3236 (NH), 1712, 1668 (3 C=O); 1H NMR (DMSO-d6) δ: 7.12-9.06 (m, 16H, ArH), 9.87 (s, 1H, NH, exchangeable with D2O); 13C NMR (DMSO-d6) δ: 119.96, 122.17, 123.46, 123.65, 125.57, 127.31, 127.68, 128.41, 129.34, 130.68, 130.85, 132.56, 135.23, 136.29, 136.81, 137.97, 145.15, 145.64, 157.37 (Ar- C), 160.08 (C=O anhydride), 165.33 (C=O amide); MS (EI, 70 eV): m/z (%): 443 [M]+. C28H17N3O3 (443.45): calcd. C 75.84, H 3.86, N 9.48; found C 75.68, H 3.72, N 9.30.
2,6-Bis-(2-phenylquinolin-4-carboxamide)pyrrolo[3,4- f]isoindole-1,3,5,7(2H,6H)-tetrone (3).
Yield 65%, m.p. 192-194°C; IR (KBr, cm-1): ν 3220 (NH), 1705, 1680 (3 C=O); 1H NMR (DMSO-d6) δ: 7.22-9.14 (m, 24H, ArH), 9.56 (s, 1H, NH, exchangeable with D2O); 13C NMR (DMSO-d6) δ: 119.84, 122.16, 123.41, 125.31, 127.35, 127.62, 129.33, 130.82, 132.58, 135.19, 135.64, 136.25, 145.21, 145.59, 157.48 (Ar-C), 164.88 (C=O anhydride), 165.17 (C=O amide); MS (EI, 70 eV): m/z (%): 708 [M]+. C42H24N6O6 (708.68): calcd. C 71.18, H 3.41, N 11.86; found C 71.05, H 3.28, N 11.70.
2,6-Bis-(2-phenylquinolin-4- carboxamide)benzo[de]isoquinolin-1,3,6,8(2H,7H) yl)tetraone (4).
Yield 53%, m.p. 219-221°C; IR (KBr, cm-1): ν 3286 (NH), 1712, 1680 (3 C=O); 1H NMR (DMSO-d6) δ: 7.26-9.15 (m, 14H, ArH), 9.78 (s, 1H, NH, exchangeable with D2O); 13C NMR (DMSO-d6) δ: 119.87, 120.48, 122.10, 123.44, 127.34, 127.61, 129.32, 130.86, 132.55, 135.15, 135.23, 136.27, 139.77, 145.18, 145.63, 157.45 (Ar-C), 158.67 (C=O anhydride), 164.89 (C=O amide); MS (EI, 70 eV): m/z (%): 758 [M]+. C46H26N6O6 (758.74): calcd. C 72.82, H 3.45, N 11.08; found C 72.64, H 3.29, N 10.95.
N-(Ribosylhydrazon)-2-phenylquinolin-4- carboxamide (5).
A mixture of hydrazide 1 (10 mmol), and D-ribos (10 mmol) with drops of glacial acetic acid were refluxed in absolute ethyl alcohol (30 ml) for 3 h, the reaction mixture was cooled to room temperature and the residue formed was clarified, washed with ethanol, dried and crystallized from ethanol to output the designate compound 5 in 57% yield, mp 164-165°C (EtOH); IR (KBr, cm-1): 3510-3436 (OH), 3190 (NH), 1682 (C=O), 1627 (CN). 1H NMR (DMSO-d6) δ: 3.86 (m, 4H, 4OH, D2O exchangeable), 4.27 (m, 1H, H-3'), 4.38 (m, 1H, H-4 '), 4.52 m (2H, H-5 ', H-5 ''), 5.10 dd (1H, H-2'), 7.11 d (1H, H-1'), 7.21-8.84 (m, 11H, ArH+=CH ), 10.28 (s, 1H, NH, D2O exchangeable). 13C NMR (DMSO-d6) δ: 63.85 (CH2OH), 65.94(CHOH), 72.19 (CHOH), 73.89 (CHOH), 119.74, 121.68, 123.45, 126.59, 127.55, 128.67, 129.78, 131.75, 134.96, 136.24, 143.82, 145.57 (Ar-C), 153.47 (=CH), 157.25 (Ar-C), 162.73 (C=O). MS (EI, 70 eV): m/z (%): 395 [M]+. Anal. Found, %: C 63.65; H 5.29; N 10.51. C21H21N3O5 Calculated, %: C 63.79; H 5.35; N 10.63.
(E)-2-Phenyl-N'-((1-(2-(pyrrolidin-orpiperidin-1- yl)ethyl)-1H-indol-3-yl)methylene) quinolin-4- carbohydrazide (6a,b) and (E)-N'-(4-substituted benzylidene)-2-phenylquinoline-4-carbohydrazide (7a-c).
General procedure: A stirred solution of hydrazide 1 (10 mmol) and appropriate aldehydes, namely, 1-(2-(pyrrolidin-1- yl)ethyl)-1H-indole-3-carbaldehyde, 1-(2-(piperidin-1- yl)ethyl)-1H-indole-3-carbaldehyde, 4- morpholinobenzaldehyde, 4-(piperazin-1-yl)benzaldehyde, or 4-piperidnobenzaldehyde (10 mmol) in acetic acid (10 ml) were refluxed for 3-5 h. The reaction mixture was allowed to cool and the obtained solid product was filtered off, dried, and crystallized from the proper solvents to give the title hydrazones respectively [17].
(E)-2-phenyl-N'-((1-(2-(pyrrolidin-1-yl)ethyl)-1Hindol- 3-yl)methylene)quinolin-4-carbohydrazide (6a).
Yield 51%, m.p.147-149°C (MeOH); IR (KBr, cm-1): ν 3195 (NH), 1684 (C=O); 1H NMR (DMSO-d6) δ: 1.56-2.30 (m, 8H, 4CH2), 2.93 (t, 2H, CH2), 3.67 (t, 2H, CH2), 7.03-9.01 (m, 15H, ArH+=CH), 9.86 (s, 1H, NH, exchangeable with D2O); 13C NMR (DMSO-d6) δ: 26.32 (pyrrol.), 49.16, 54.01 (CH2), 57.58 (pyrrol.), 110.23, 112.34, 118.36, 119.74, 120.63, 122.18, 122.88, 123.42, 126.28, 127.30, 127.51, 129.34, 130.75, 132.52, 134.75, 134.87, 135.94, 136.49, 143.19, 145.21, 145.68, 157.47 (Ar-C), 163.30 (C=O); MS (EI, 70 eV): m/z (%): 487 [M]+. C31H29N5O (487.59): calcd. C 76.36, H 5.99, N 14.36; found C 76.19, H 5.81, N 14.22.
(E)-2-phenyl-N'-((1-(2-(piperidin-1-yl)ethyl)-1Hindol- 3-yl)methylene)quinolin-4-carbohydrazide (6b).
Yield 53%, m.p. 176-178°C (AcOH/H2O); IR (KBr, cm-1): ν 3220 (NH), 1678 (C=O); 1H NMR (DMSO-d6) δ: 1.53-2.21 (m, 10H, 5CH2), 2.89 (t, 2H, CH2), 3.72 (t, 2H, CH2), 6.98-9.01 (m, 15H, ArH+=CH), 9.54 (s, 1H, NH, exchangeable with D2O); 13C NMR (DMSO-d6) δ: 25.36 , 25.71 (piperid.), 48.12, 53.69 (CH2), 56.01(piperid.), 109.20, 111.86, 118.55, 119.78, 120.59, 122.15, 122.92, 123.48, 126.65, 127.32, 127.63, 129.30, 130.87, 132.56, 134.44, 134.95, 135.80, 136.51, 143.22, 145.16, 145.74, 157.42 (Ar-C), 163.87 (C=O); MS (EI, 70 eV): m/z (%): 502 [M]+. C32H31N5O (501.62): calcd. C 76.62, H 6.23, N 13.96; found C 76.47, H 6.11, N 13.79.
(E)-N'-(4-(morpholin-1-yl)benzylidene)-2- phenylquinoline-4-carbohydrazide (7a).
Yield 56%, m.p. 190-192°C (MeOH); IR (KBr, cm-1): ν 3210 (NH), 1680 (C=O), 1613(C=N) ; 1H NMR (DMSO-d6) δ: 2.98 (t, 4H, CH2NCH2), 3.79 (t, 4H, CH2OCH2), 6.90-8.86 (m, 15H, ArH+=CH), 10.36 (s, 1H, NH, exchangeable with D2O); 13C NMR (DMSO-d6) δ: 49.96, 67.12 (morpholin-C), 115.28, 119.65, 121.59, 122.89, 123.56, 126.82, 127.48, 128.94, 129.87, 131.09, 133.15, 134.76, 135.89, 143.26, 144.90, 145.58, 152.14, 158.03 (Ar-C), 165.21 (C=O); MS (EI, 70 eV): m/z (%): 436 (8) [M]+. C27H24N4O2 (501.62): calcd. C 74.29, H 5.54, N 12.84; found C 74.12, H 5.39, N 12.71.
(E)-N'-(4-(piperazin-1-yl)benzylidene)-2- phenylquinoline-4-carbohydrazide (7b).
Yield 51%, m.p. 177-179°C (MeOH); IR (KBr, cm-1): ν 3195 (NH), 1668 (C=O), 1610(C=N) ; 1H NMR (DMSO-d6) δ: 2.86 (t, 4H, CH2NCH2), 3.09 (t, 4H, CH2NCH2), 6.85-8.84 (m, 15H, ArH+=CH), 10.45, 11.05 (2s, 2H, 2NH exchangeable with D2O); 13C NMR (DMSO-d6) δ: 47.13, 53.29 (piperazin- C), 115.10, 120.34, 121.74, 123.17, 123.67, 127.14, 127.55, 129.05, 130.28, 131.12, 133.25, 135.06, 136.42, 143.18, 145.03, 145.73, 152.23, 157.79 (Ar-C), 164.87 (C=O); MS (EI, 70 eV): m/z (%): 435 (12) [M]+. C27H25N5O (435.52): calcd. C 74.46, H 5.79, N 16.08; found C 74.32, H 5.65, N 17.93.
(E)-N'-(4-(piperidin-1-yl)benzylidene)-2- phenylquinoline-4-carbohydrazide (7c).
Yield 60%, m.p. 193-195°C (MeOH); IR (KBr, cm-1): ν 3236 (NH), 1682 (C=O), 1628(C=N) ; 1H NMR (DMSO-d6) δ: 1.47 (m, 6H, 3CH2), 3.01 (t, 4H, CH2NCH2), 6.87-8.84 (m, 15H, ArH+=CH), 10.26 (s, 1H, 1NH exchangeable with D2O); 13C NMR (DMSO-d6) δ: 24.86, 25.43, 53.16 (piperidin-C), 115.23, 119.88, 121.95, 123.10, 123.79, 126.98, 127.64, 128.87, 139.83, 131.07, 133.20, 134.75, 135.67, 143.12, 144.92, 145.69, 153.01, 158.11(Ar-C), 165.33 (C=O); MS (EI, 70 eV): m/z (%): 434 (6) [M]+. C28H26N4O (434.53): calcd. C 77.39, H 6.03, N 12.89; found C 77.28, H 5.89, N 12.74.
Pharmacological assay
Experimental animals: Healthy male adult Wister albino rats, weighing approximately 250 g, were used. They were housed in polyethylene cages and were kept at a constant temperature (22 ± 2°C), humidity (55%) and 12 h light-dark conditions. The animals were provided with Purina chow rat diet and free access to drinking water.
Carrageenan-induced edema
The carrageenan-induced rat hind paw edema test was conducted as described previously [20]. For each compound rat received treatment with 2 doses 40 mg/kg and 80 mg/kg (intraperitoneal injection) one hour before carrageenan 2% injection into hind paw of the rats. The paw volume was estimated with the help of plethysmometer 7140 (UGO Basile 7140, model-7141, Biological research apparatus, Italy) at zero time (before carrageenan injection), 1 and 2 hours post injection. Then all results tabulated and the percent inhibition in the rat paw calculated using the following formula:
% inhibition = (dc-dt)/dc × 100
Where
“dt” is the difference in paw volume in the treated group.
“dc” the difference in paw volume in the control group.
“ED50” for each treatment period was also calculated and expressed in micromole/kg for comparing potencies of given compounds.
Peripheral analgesic activity (writhing test).
The peripheral analgesic activity of selected compounds was determined in mice as described in the work done by Jayanna et al. [21]. Seventy two mice were divided into 12 groups of 6 animals each. Mice of groups 1 (normal control) and 2 (reference) were treated orally with the vehicle (5 ml/kg) and acetyl salicylic acid (150 mg/kg), respectively. Animals of groups 3 through 12 were orally given selected compounds at doses of (20 mg/kg, p.o.). After 30 min of medication, writhings were induced by an intraperitoneal injection of acetic acid (0.7% aqueous solution) in a dose of 10 ml/kg. Mice were then placed in transparent boxes and the number of writhes per animal was counted for 20 min after acetic acid injection and expressed as the percentage of protection using the following ratio:
Protection (%) = [(Control mean - Treated mean)/Control mean] × 100.
Results and Discussion
Anti-inflammatory activity
The anti-inflammatory activities of the newly synthesized compounds was estimated by carrageenan-induced paw edema model in rats [20] utilizing Diclofenac sodium as a standard drug. The anti-inflammatory activity results (Table 1) according to structure-activity relationship (SAR) indicated that, compound 5 possessing sugar substitution on 2-phenyl quinoline moiety confers considerable anti-inflammatory activity followed by compound 2 bearing imido quinoline-4- carboxamide substitution, then the activity decreased in compounds 6a, 7a, 4, 3, 7b, 7c and 6b in descending order in 1 hour treatment time with respect to diclofenac sodium. In 2 hours treatment time, compounds 5 possessing glycoside shiff,s base substituted on 2-phenyl quinoline moiety showed higher activity as that of diclofenac sodium (reference drug) followed by compound 2 bearing imido quinoline-4- carboxamide substitution, then compound 4 bearing bis-imido quinoline-4-carboxamide substitution. The rest of the compounds 6a, 3, 7a, 7b and 7c showed the least inhibitory effect in descending order. The high activity of the glycoside function may be due to the presence of the free hydroxyl functional groups in the molecules structure.
| Compound ID | % inhibition after 1 hour | % inhibition after 2 hour | ED50 1 hour treatment mg/kg | ED50 2 hours treatment mg/kg | ED50 1 hour treatment μM/kg | ED50 2 hours treatment μM/kg | ||
|---|---|---|---|---|---|---|---|---|
| Dose 40 mg/kg | Dose 80 mg/kg | Dose 40 mg/kg | Dose 80 mg/kg | |||||
| Control | 0.83 | 1.03 | - | - | - | - | ||
| Diclofenac sodium | - | - | - | - | 17 | 53.4 | ||
| 2 | 59.03 | 91.5 | 70.8 | 100 | 32.9 | 24.4 | 74.2 | 55.02 |
| 3 | 42.1 | 68.6 | 0 | 114.4 | 49.2 | 54.1 | 69.4 | 76.3 |
| 4 | 60.2 | 78.3 | 46.6 | 78.6 | 27.1 | 43.1 | 35.7 | 56.8 |
| 5 | 100 | 91.5 | 68.9 | 84.4 | 5942.5 | 17.2 | - | 53.7 |
| 6a | 80.7 | 57.8 | 55.3 | 66.9 | 100.3 | 29.1 | 255 | 74 |
| 6b | 50.6 | 73.4 | 37.8 | 44.6 | 39.3 | 192.6 | 129 | - |
| 7a | 38.5 | 91.5 | 47.5 | 86.4 | 46.5 | 41.8 | 87.5 | 78.7 |
| 7b | 3.61 | 91.5 | 0 | 66.01 | 57.7 | 67.6 | 147.04 | 172.3 |
| 7c | 67.4 | 32.5 | 73.7 | 50.4 | 56.5 | 80.95 | 132.8 | 190.3 |
Table 1. Effect of the synthesized compounds on carrageenan induced paw edema in rats.
Analgesic activity
Analgesic activity of the selected compounds was carried out in mature Albino mice (27 30 g b.wt) by using Peripheral analgesic activity (writhing test).
The tested compounds were shown to produce variable analgesic activity in the writhing assays in mice. The selected compounds showed significant reduction in the number of writhes (Table 2). Compound 5 displayed high inhibition rate in acetic acid-induced writhing test.
| Compounds | No. of writhes | Percentage of inhibition (%) |
|---|---|---|
| Control | 31.5 ± 2.63 | - |
| 2 | 34.5 ± 3.48** | 3.28 |
| 3 | 28.7 ± 4.23* | 7.01 |
| 4 | 38.9±2.90** | -13.54 |
| 5 | 19.5±1.78** | 44.37 |
| 6a | 32.8 ± 2.95* | 10.56 |
| 6b | 25.4±1.10* | 18.38 |
| 7a | 29.1±2.05* | 29.18 |
| 7b | 30.5 ± 3.17** | 11.89 |
| 7c | 22.8±1.86* | 6.55 |
| Aspirin | 19.0 ± 2.58 ** | 43.28 |
*P<0.05, **P<0.01 versus control group.
All drugs were dissolved in DMSO (20 mg/kg, orally), except acetyl salicylic acid (was dissolved in DW, 150 mg/kg, orally).
Table 2. Peripheral analgesic activity in mice (Writhing test) of the synthesized compounds on mice.
Chemistry
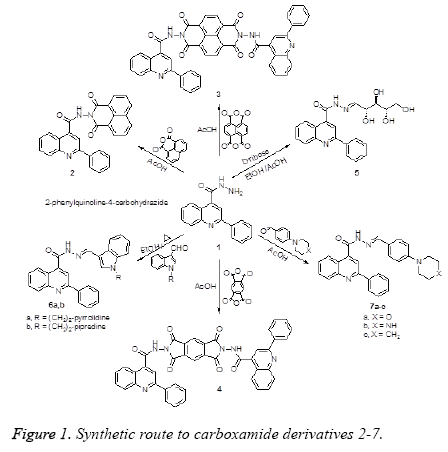
The synthetic pathway leading to the title products is outlined in Figure 1. The starting hydrazide 1 was treated with dicarboxylic acid anhydride and tetracarboxylic acid dianhydrides namely, 1,8-naphthalenedicarboxylic acid anhydride and benzene or naphthalene tetracarboxylic acid dianhydride to afford the corresponding imide and bis-imide derivatives 2, 3 and 4, respectively. Reaction of 1 with sugar aldose and aryl aldehydes namely, D-ribose, 1-(2-(pyrrolidin-1- yl)ethyl)-1H-indole-3-carbaldehyde, 1-(2-(piperidin-1-yl)ethyl) -1H-indole-3-carbaldehyde, 4-(morpholin-1-yl)benzaldehyde 4-(piperazin-1-yl)- benzaldehyde and 4-(piperidin-1-yl)benzaldehyde afforded the corresponding Schiff bases 5, 6a,b and 7a-c, respectively (Figure 1).
Conclusion
The significance of the potent quinoline derivatives has been established in the search for effective anti-inflammatory agents. In the current study, a new scaffold of quinoline linked to carboxamides were synthesized and evaluated for their analgesic and anti-inflammatory agents. The results of this investigation revealed that, anti-inflammatory activity of quinoline substituted different nitrogenous heterocycles on carrageenan-induced rat paw edema model identified compound 5 as a potent anti-inflammatory agent showed significant anti-inflammatory activity as that of diclofenac sodium (standard drug). Therefore, this derivative may represent good lead compound for the development of potent and selective anti-inflammatory agents.
Acknowledgement
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
References
- Said AE, Zakaria KA, Nermine AO, Jasmine L, Mohamed AK, Hamdy KT. Synthesis, molecular docking and anti-inflammatory screening of novel quinoline incorporated pyrazole derivatives using the Pfitzinger reaction II. Bioorg Chem 2015; 58: 104-116.
- Sharad KS, Varun J, Sandeep L, Sumit B, Anil C, Amit T, Angel TA, Alex J. Novel quinolone substituted thiazolidin-4-ones as anti-inflammatory, anticancer agents: Design, synthesis and biological screening. Eur J Med Chem 2013; 63: 589-602.
- Said AE, Hamdy KT, Mustafa TU. Synthesis, molecular modeling and anti-inflammatory screening of novel fluorinated quinoline incorporated benzimidazole derivatives using the Pfitzinger reaction. J Fluorine Chem 2014; 161: 87-94.
- Rajanarendar E, Nagi Reddy M, Rama Krishna S, Rama Murthy K, Reddy N, Rajam V. Design, synthesis, antimicrobial, anti-inflammatory and analgesic activity of novel isoxazolyl pyrimido[4,5-b]uinolones and isoxazolyl chromeno[2,3-d]pyrimidin-4-ones. Eur J Med Chem 2012; 55: 273-283.
- N’Da D, Breytenbach C, Smith J, Lategan C. Synthesis, cytotoxicity and antimalarial activity of ferrocenyl amides of 4-aminoquinolines. Arzneimittel forschung 2010; 60: 627-635.
- Eswaran S, Adhikari AV, Shetty NS. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur J Med Chem 2009; 44: 4637-4647.
- Desai NC, Dodiya AM. Synthesis, characterization and in vitro antimicrobial screening of quinoline nucleus containing 1,3,4-oxadiazole and 2-azetidinone derivatives. J Saudi Chem Soc 2014; 18: 425-431.
- Desai NC, Maheta AS, Rajpara KM, Joshi V, Vaghani HV, Satodiya HM. Green synthesis of novel quinoline based imidazole derivatives and evaluation of their antimicrobial activity. J Saudi Chem Soc 2014; 18: 963-971.
- Pankaj BM, Mahesh AB, Anand AM. Synthesis and biological evaluation of 1-(5-(2-chloroquinolin-3-yl)-3-phenyl-1Hpyrazol-1-yl)ethanone derivatives as potential antimicrobial agents. J Saudi Chem Soc 2015; 19: 655-660.
- Gakhar G, Ohira T, Shi A, Hua DH, Nguyen TA. Antitumor effect of substituted uinolones in breast cancer cells. Drug Develop Res 2009; 69: 526-534.
- Xiao-Qin W, Chun-Li X, Shuo-Bin C, Jia-Heng T, Tian-Miao O, Shi-Liang H, Ding L, Lian-Quan G, Zhi-Shu H. Design, synthesis, and biological evaluation of 2-arylethenylquinoline derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem 2015; 89: 349-361.
- El-Gazzar AB, Youssef MM, Youssef AM, Abu-Hashem AA, Badria FA. Design and synthesis of azolopyrimidoquinolines, pyrimidoquinazolines as anti-oxidant, anti-inflammatory and analgesic activities . Eur J Med Chem 2009; 44: 609-624.
- Sun XY, He XJ, Pan CY, Liu YP, Zou YP. Synthesis and study of the antidepressant activity of novel 4,5-dihydro-7-alkoxy(phenoxy)-tetrazolo[1,5-a]quinoline derivatives. Med Chem Res 2012; 21: 3692-3698.
- Skoda-F€oldes R, Kollar L. Synthetic applications of palladium catalyzed carbonylation of organic halides. Curr Org Chem 2002; 6: 1097-1119.
- Vivek K, Manu J, Anu TS, Alka M, Vinod S, Pratibha S, Pramod K, Raghuveer I, Anand CB. 1,8-Naphthyridine-3-carboxamide derivatives with anticancer and anti-inflammatory activity. Eur J Med Chem 2009; 44: 3356-3362.
- Khalifa NM, Al-Omar MA, Amr AE, Baiuomy AR, Abdel Rahman RF. Synthesis and biological evaluation of some novel fused thiazolo[3,2-a]pyrimidines as potential analgesic and nti-inflammatory agents. Russ J Bioorg Chem 2015; 41: 192-201.
- Eweas AF, Khalifa NM, Ismail NS, Al-Omar MA, Soliman AM. Synthesis, molecular docking of novel 1,8-naphthyridine derivatives and their cytotoxic activity against HepG2 cell lines. Med Chem Res 2014; 23: 76-86.
- Almutairi MS, Hegazy GH, Haiba ME, Ali HI, Khalifa NM, Soliman A. Synthesis, Docking and Biological Activities of Novel Hybrids Celecoxib and Anthraquinone Analogs as Potent Cytotoxic Agents. A.M. Int J Mol Sci 2014; 15: 22580-22603.
- Khalifa N, Naglah A, Al-Omar M , Abo-Ghalia M, Amr A. Synthesis and reactions of new chiral linear carboxamides with an incorporated peptide linkage using nalidixic acid and amino acids as starting materials. Z Naturforsch 2014; 69: 351-361.
- Kumar V, Bhat ZA, Kumar D, Khan NA, Chashoo IA. Evaluation of anti-inflammatory potential of leaf extracts of Skimmia anquetilia. Asian Pac J Trop Biomed 2012; 2: 627-630.
- Jayanna ND, Vagdevi HM, Dharshan JC, Raghavendra R, Telkar SB. Synthesis, antimicrobial, analgesic activity, and molecular docking studies of novel 1-(5,7-dichloro-1,3-benzoxazol-2-yl)-3-phenyl-1H-pyrazole-4-carbaldehyde derivatives. Med Chem Res 2013; 22: 5814-5822.