- Biomedical Research (2012) Volume 23, Issue 3
Anti-hypercholesterolemic activity of Juniperus communis Lynn Oil in rats: A biochemical and histopathological investigation
Akdogan M1, Koyu A2, Ciris M3, Yildiz K4
1Department of Medical Biochemistry, Faculty of Medicine, Sakarya University, Sakarya, Turkey
2Department of Medical Physiology, Faculty of Medicine, Suleyman Demirel University, Isparta, Turkey
3Department of Medical Patholgy, Faculty of Medicine, Suleyman Demirel University, Isparta, Turkey
4Department of Public Health, Faculty of Medicine, Suleyman Demirel University, Isparta, Turkey
- Corresponding Author:
- Mehmet AKDOGAN
Sakarya University, School of Medicine
Departments of Biochemistry
54055 - Sakarya,
Turkey
Accepted date: March 13 2012
Abstract
The aromatic fruit, its extract and the oil of Juniperus Communis Lynn (JCL), a tree species that grows in the Mediterranean and Isparta region, have been commonly used at least for three centuries as herbal treatment and in the field of medicine. The aim of this study was to apply JCL in various doses to rats in the treatment of hypercholesterolemy and analyse the biochemical parameters and the hystopathologic effects on kidney tissue. In this study 35 adult male Wistar albino rats of 200-250 gr in weight were used. The rats were divided into 5 equal groups of 7. The control group was fed with normal pellet chow, the cholesterol (Chol) group was fed with pellet chow containing 2% of cholesterol, and the 50 JCL, 100 JCL and 200 JCL groups were fed with 50, 100, 200 mg/kg JCL oil, respectively in addition to the 2% cholesterol-containing pellet chow. JCL was dissolved in 0.5% Sodium Carboxy Methyl Cellulose (SCMC) and administered by a gavage needle. The experiment was ended 30 days later and blood and kidney tissue samples were taken. A complete blood test and biochemical measurements were made. Kidney tissues were analysed histopathologically. The administration of 200 mg/kg JCL led to a significant increase in BUN and Crea levels. The administration of chol increased the TC level significantly, but the administration of JCL together with chol prevented this increase. The administration of chol led a significant increase in Ox-LDL levels. However, the administration of 200 mg/kg JCL together with chol did not result in a significant increase. At the end of the study it was found that the JCL oil showed an anti-hypercholesterolemic effect. While no anemic effect and no distinct change overall were observed in all groups, a distinct focal damage was observed in the tubular cast structure.
Keywords
Juniperus Communis Lynn, anti-hypercholesterolemic activity, renal toxicity.
Introduction
The common juniper tree (Juniperus Communis Lynn), the leaves of which remain green throughout the four seasons, is one of the juniper tree species found in the northern hemisphere. The aromatic fruit and oil of the juniper has been used in herbal treatment and in the field of medicine for at least three centuries. The fruit of the juniper tree contains 2% volatile oil and 10% balm. There are more than 100 constituents in the fruit’s volatile oil. Among these are monoterpene derivatives such as alpha and beta, mirsene, limonene, sabinene and terpinine-4-ol, which is an alcohol derivative. In addition, in the juniper fruit there is approximately 30% sugar, a low amount of catechic tannin, flavonoid derivatives and leuco antosiyanines [1-4]. In the past, the diuretic effect of the juniper fruit was traditionally benefitted from in healing kidney and bladder disorders. The diuretic effect of juniper oil has been revealed and this effect has been attributed to terpinine-4-ol, which increases the glomerular filtration rate in the kidneys [5]. More recently, juniper oil has also been administered in disorders such as rheumatism and arthritis. In-vitro studies reported that the extract of juniper fruit could prevent prostaglandins; this indicates that there is a scientific basis to the relaxing effect of juniper in disorders such as rheumatoid arthritis. In addition, juniper extract also prevents platelet activating factor (PAF) and, thus, increases the blood clotting time. With these features, the juniper takes its place among the herbal treatment methods [1-3]. However, the intake of the juni per fruit in high doses (30 g/day and above) for a month or longer causes irritation in the gastrointestinal system and the kidneys, and these undesired effects have been linked to high rates of hydrocarbons and low amounts of terpinine-4-ol [6]. The other characteristics of this plant that are of benefit are its nausea reliever effects. Juniper fruit also has an antioxidant feature. It has been found that this feature derives from the limonene gamma-terpinene, caryophyllene, germacren D, alpha humulin present in the fruit [7-10].
It has been revealed that juniperus fruit extract has antiherpetic [11], antimicrobial [12-14], antifungal [15] and anticancerogene effects and that it can be used in the treatment of both liver and colon cancer and myosarcoma [17,18]. Researchers’ in-vivo studies [19] reported that limonene found in juniperus extract prevented LDL oxidation and, thus, could slow down atherosclerotic progression. In addition, it increases the effect of lithium, so it should not be used together with lithium [23].
The role that hypercholesterolemy plays in the pathogenesis of coronary heart disease (CAD) has clearly been revealed and it has been proved with numerous clinical studies that lowering serum cholesterol with particularly HMG-CoA Reductase enzyme inhibitors, that is statins, reduces coronary problems. By inhibitting the HMG-CoA Reductase enzyme, which is a rate-limiting enyzme for cholesterol synthesis, statines increase the number of LDL receptors in the lungs and enable the intake of LDL cholesterol from the plasma to the liver [24, 25]. However, because they are primarily metabolized in the liver, one must be careful in terms of liver toxicity [26, 27]. It has been reported that statins can result in side effects of increasing ALT and AST at the rate of 2-5% [28].
In some studies, it has also been reported that the isoprenoids in JCL oil has hypolipidemic effects [29]. JCL oil, the subject of the study, can be more advantageous in that it is both economical and has no side-effects caused by statins.
In this study, we aimed to determine the anemic, antihypercholesterolemic and nefrotocsic effects on hematologic and biochemical parameters and kidney tissues of rats by analysing the hystopathologic effects of the oil extracted from JCL fruit grown in Isparta and the Mediterranean region by means of hydrodistillation.
Materials and methods
This study was carried out in Suleyman Demirel University, Faculty of Medicine, the Experimental Animal Laboratory. Prior to this study, the protocol was reviewed and approved by the Suleyman Demirel University Ethics Commitee for Animal Research.
Experimental Procedure
In this study, 35 adult male Wistar albino rats of weights ranging between 200-250g, obtained from Suleyman Demirel University, Experimental Animal Laboratory, were used. The rats were divided into 5 groups of 7. The Control group was fed normal pellet chow, the cholesterol (Chol) group was fed pellet chow containing 2% cholesterol and the 50 JCL, 100 JCL and 200 JCL groups were fed using a 4 ml gavage needle with pellet chow containing 2% cholesterol in addition to 50, 100 and 200 mg/kg JCL oil dissolved in 0.5% Sodium Carboxy Methyl Cellulose (SCMC). The rats were kept under standard light (12 hours of day light/12 hours of darkness) and at a temperature of 25 °C. They were fed 30 days in specially prepared cages.
The rats were kept under standard light (12 hours of day light/12 hours of darkness) and at a temperature of 25 °C. They were fed 30 days in specially prepared cages. 30 days after the beginning of the study, the experiment was ended with the collection of blood samples and kidney tissues under ketamine anesthesia. The kidneys for pathological study were fixed in a 10% formalin (pH 7.4 Phosphate buffer) solution.
Materials
JCL oil
In the study, the rats received 50, 100, 200 mg/kg doses of Juniperus communis Linn. oil (produced via the distillation method by Semasen Baharat-Arsen, a Food, Drugs and Cosmetics Firm in Isparta). A 4 ml.-mixture of JCL was dissolved in 0.5% Sodium Carboxy Methyl Cellulose and administered.
Oil obtained from JCL fruit by means of hydroxilation method was found to compose of monoterpene hydrocarbon (22.97%), oxygenated monoterpene (14.86%), sesquiterpene hydrocarbon (32.43%), oxygenated sesquiterpene (6.75%), diterpene (1.35%), and others (21.62%) [30].
Biochemical Analysis
The preparation of the Serum Samples
At the end of 30 days, all groups were decapitated under anesthesia (Ketamine 90 mg/kg + Xylazine 10 mg/kg). Approximately 6-7 ml of blood samples from the abdominal aorta were collected into standard tubes, citrate tubes and EDTA tubes placed in ice. While the tubes without anticoagulant and the citrate tubes were centrifuged at 4000 rpm for 10 minutes at room temperature, the EDTA tubes were centrifuged in a cooling centrifuge. The serum from the tube without anticoagulant was analysed for levels of glucose, BUN, creatine, total protein, albumin, I, TIBC, ALP, ALT, AST, ALP, LDH, TC, and HDL-C, LDL-C, TG on the same day using an Olympus AU2700 autoanalyser (Japan) and Olympus brand com mercial kit. The number of leukocytes (WBC), the number of erythrocytes (RBC), Haemoglobin (HGB), Hematocrit (HTC), Mean corpuscular volume (MCV), Mean corpuscular hemoglobin (MCH), Mean corpuscular hemoglobin concentration (MCHC) and Platelet (PLT) values in blood were measured with EDTA by using Coulter.
MAX-M autoanalyser.
Oxide-LDL Immune Diagnostic AG was measured by using the D64625 Bensheim brand Ox-LDL ELISA kit and the ELX808 Ultra Microplate Reader (USA) brand ELISA device.
Histopathological Examination
The kidney tissue specimens taken from the rats were fixed in a 10% formalin (pH 7.4 Phosphate buffer) solution. After routine follow-ups, 5-6 micron thick cross sections were taken and stained with hematoxylin-eosin dye. Cross sections in 5-6 micron of thickness were taken from the kidney. After the slides were stained with Hematoxylin- Eosin and Masson’s Trichrome, they were evaluated using the Olympus BO 71 light microscope.
Statistical Evaluation
Statistical analyses were performed using the SPSS for Windows Version 9.05 program. The “Kruskal-Wallis Variance analysis” test was used to determine whether there was a significant difference among the groups in general. The pairwise comparisons were conducted using the “Mann-Whitney U” test. The significance level was accepted as p<0.05. The results were reported as mean ± SD values.
Results
Haematologic Tests
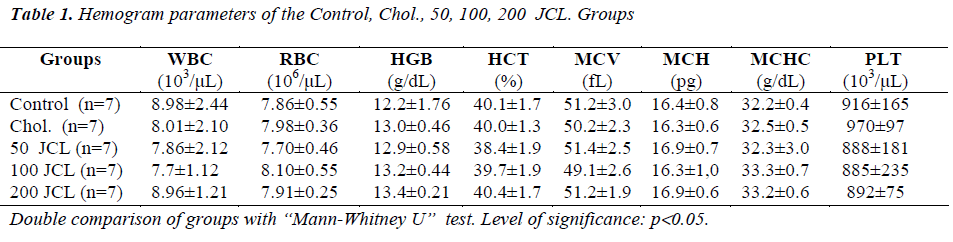
When the WBC, RBC, HGB, HTC, MCV, MCH, MCHC and PLT values belonging to the Chol., 50, 100 and 200 JCL groups were compared with those of the control group, no statistically significant difference was observed (p>0.05) (Table 1).
Biochemical Tests
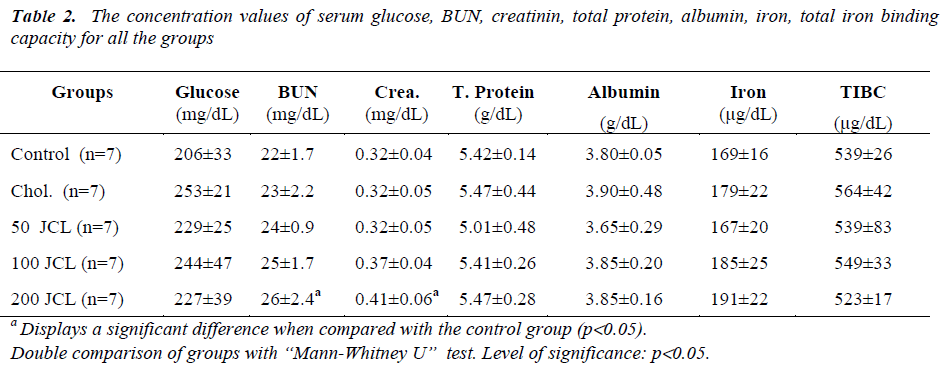
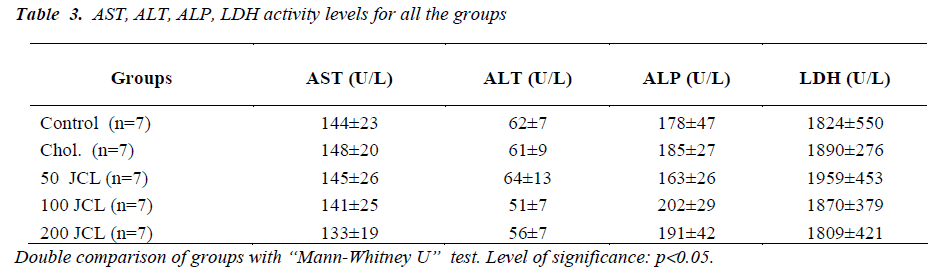
When the glucose, total protein, albumine, I, TIBC concentrations of the Chol., 50, 100 and 200 JCL groups and the AST, ALT, ALP and LDH activity levels were compared with the control group, no statistically significant difference was observed (p>0.05) (Tables 2). While no significant difference was found when the BUN and Crea. concentrations of the Chol., 50 and 100 JCL groups were compared (p>0.05), comparatively the increase in the BUN and Crea. concentration of the 200 JCL group was found significant (p<0.05) (Table 2).
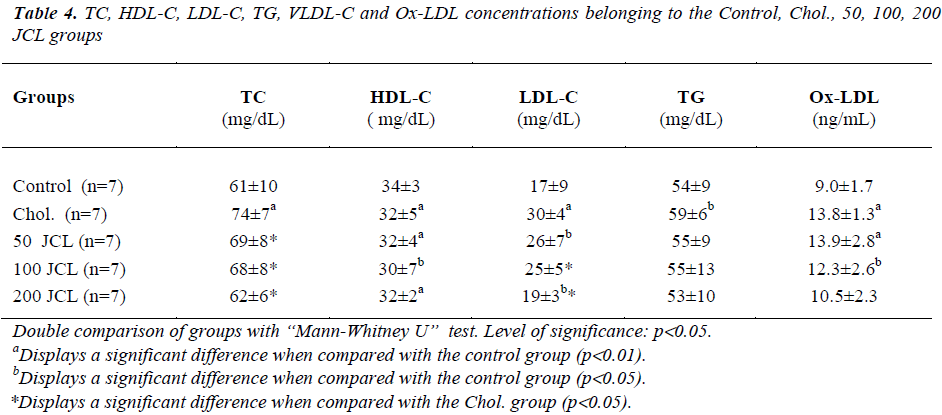
There was a significant increase in the TC concentrations of the Chol. group when compared with those of the control group (p<0.01); however, the increase in the TC concentrations of the 50, 100 and 200 JCL groups were not found to be significant (p>0.05) (Table 4). The TC concentrations of the Chol. group, when compared with those of the 50, 100 and 200 JCL groups, were found to be significantly low (p<0.05), (Table 4).
A significant decrease in the HDL-C concentrations of the Chol., 50, 100 and 200 JCL groups was observed when compared to those of the control group (p<0.01, p<0.01, p<0.05, p<0.01, respectively). However, the decrease in the HDL-C concentrations of the 50, 100 and 200 JCL groups were not significant when compared with the Chol. group (p>0.05) (Table 4).
The increase in the LDL concentrations of the Chol., 50 and 200 JCL groups was found significant when compared with the control group ( p<0.01, p<0.05, p<0.05, respectively). In addition, the increase in the LDL concentration of the 100 JCL group was not found to be statistically significant when compared with the control group (p>0.05) (Table 4).
While there was a significant increase in the TG concentrations of the Chol. group when compared with the control group (p<0.05), the increase observed in the 50 , 100 and 200 JCL groups was not significant (p>0.05). When compared with the Chol. group, the decrease in the TG concentrations of the 50, 100 and 200 JCL groups was not found significant (p>0.05) (Table 4).
The increase in the Ox-LDL concentrations of the Chol., 50, and 100 JCL groups were found to be significant when compared with the control group (p<0.01, p<0.01, p<0.05, respectively), but the increase in the Ox-LDL concentration of the 200 JCL group was not found significant when compared with the control group (p>0.05).
Histopathological Examination
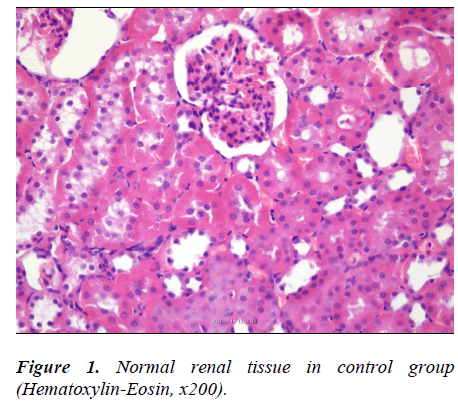
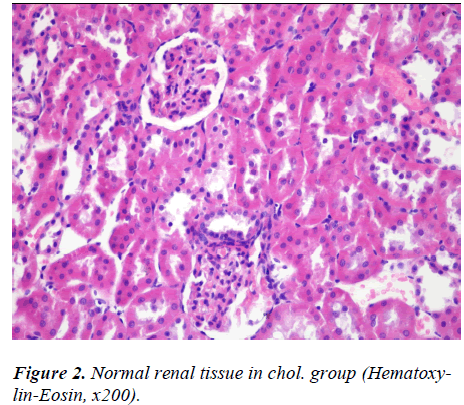
There was no abnormal finding in the sections from kidney in Control, Chol. and 50 JCL groups (Fig. 1-3).
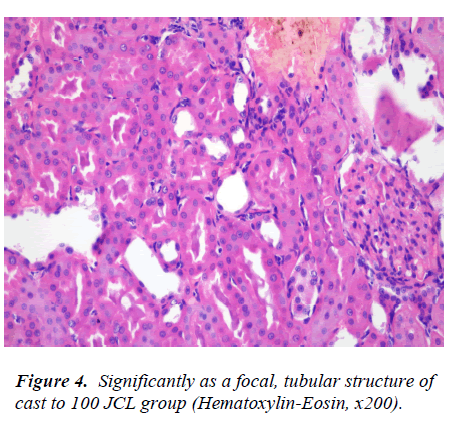
The presence of focal significant tubular cast structures and the absence of other findings in the sections from kidney in 100 JCL and 200 JCL groups (Fig. 4-5).
Discussion
High blood cholesterol levels are one of the fundamental reasons underlying serious health problems, primarily atherosclerosis and heart diseases, of people today. For this reason, retaining the blood cholesterol levels at normal levels is important to stop the progression of numerous diseases.
The production of reliable and more effective drugs is the responsibility of the scientific fields of pharmacology and pharmacy. Today, numerous drugs to balance the blood cholesterol levels have been released to the market and are being used.
HMG Co-A reductase inhibitors (statins), lipid-lowering agents, are commonly used to reduce blood lipid levels and to treat cardiovascular disease [31]. Butani et al. [6] have reported that high doses of juniper fruit causes irritation in the gastrointestinal system and particularly in the kidneys of rats.
In our study, the anemic, anti-hypercholesterolemic and the histopathologic effect of oil extracted from the JCL fruit grown in Isparta and the Mediterranean region on the rats fed with a controlled diet containing 2% cholesterol were studied by analysing the rats’ hematologic and biochemical parameter levels and the histopathological effects on their kidney tissue. Our study is the first experimental study in literature studying the hematologic, biochemical and nephrotoxicity effect of JCL oil in varying doses on rats.
When the hematologic parameters of the blood samples were examined, no difference across the groups was observed in terms of the WBC, RBC and PLT numbers and HGB, HTC, MCV, MCH, and MCHC levels.
Studies conducted on animals revealed that the experimentally established diabete models of the JCL fruit reduced blood sugar. However, this effect has not been experimented on human beings [31-34]. In our study, JCL oil was used instead of JCL fruit and it was found that it does not change serum glucose levels.
In our study, measurements were made of serum Total protein, Albumin, Iron (I), Total Iron Bindinc Capacity (TIBC), Alanine aminotrasferase (ALT), Aspartate aminotrasferase (AST), Alkaline phosphatase (ALP), Lactate dehydrogenase (LDH) using the rat blood samples. In these measurements no significant difference was found between the treatment groups and the control and Chol. groups. Moreover, the varying doses of JCL oil did not create a different effect on the above-mentioned biochemical parameters. On the other hand, while the increase in the BUN and Crea. concentrations of the 50 and 100 JCL groups were not found significant when compared with the control group, the increase in the 200 JCL group was found significant. This finding indicates that administering a high dose of JCL oil can impair kidney function.
In some studies it was found that juniper fruit had an antihypercholesterolemic effect [34, 35]. However, we believe that the JCL oil used in this study is more advantageous because of both its low cost and the non-presence of side effects that can be caused by statins. In some studies, it was also reported that isoprenoids have hypolipidemic effect [29]. Williams et al. [36] report that there are numerous mechanisms in which plant sterols and stanols prevent the absorption of and thus reduce cholesterol. They listed some of the most important ones as follows:
1. Plant sterol competes with cholesterol to unite with micelle thus leading to lower cholesterol absorption;
2. Plant sterol competes with cholesterol to enter through the cell membrane in the intestines, thus leading to low cholesterol absorption; 3. The soluble cholesterol transforms into non-soluble cholesterol, which prevents absorption. In this way, cholesterol is excreted through feces.
In this study, administering a diet containing 2% cholesterol significantly increased the TC, LDL-C concentrations. Adding 50, 100 and 200 mg/kg doses of JCL oil to their diet containing 2% cholesterol significantly decreased the TC, LDL-C concentrations when compared with administering diets only containing 2% cholesterol.
While there was a significant decrease in the HDL-C concentrations of the Chol., 50, 100 and 200 JCL groups when compared with the control group, the decrease in the HDL-C concentrations of the 50, 100, and 200 JCL groups was not found to be significant when compared with the Chol. group.
While the increase in the LDL concentrations of the Chol., 50 and 200 JCL groups were found significant when compared with the control group, the increase in the LDL concentration of the 100 JCL group was not found significant when compared with the control group.
Administering a diet containing 2% cholesterol increased the TG concentration of the Chol. group. The TG concentrations of the 50, 100 and 200 JCL groups were found low when compared with the Chol. group. However, this decrease is not statistically significant.
Administering a diet containing 2% cholesterol in the study increased the ox-LDL level. Adding a high dose of JCL oil together with cholesterol to the diet decreased the ox-LDL level depending on the dose. This effect could derive from the antioxidant feature of the gamma terpinene, alpha and beta-pinenine found in the juniper extract as well. It has already been stated that the antioxidants display their hypocholesterolemic effects by decreasing lipid peroxidation and increasing antioxidant enzyme activity [9].
In conclusion, this study showed that the JCL oil, which was administered in 50, 100 and 200 mg/kg doses, could be effective in hypercholesterolemy. While no anemic effect was observed in all the groups, only a distinct, focal damage in the tubular cast structure in the kidneys of the group in which 200 mg/kg JCL was administered was observed.
We believe that in the near future medical plants will be in the foreground in the treatment of many diseases, primarily heart diseases, and that the need for these plants will increase. We believe that our study should lead to further studies and that more comprehensive studies need to be carried out to reveal particularly the minimal side effects on the kidney in order to benefit from the antihypercholesterolemic effect of varying doses of JCL oil.
References
- ESCOP. Juniperi fructus: Monographs on the Medicinal Uses of Plant Drugs. Exeter, U.K.: European Scientific Cooperative on Phytotherapy 1997.
- Newall C, Anderson LA, Phillipson JD. Herbal Medicines: A Guide for Health-Care Professionals. London, England: PharmaceuticalPress; 1996: 176.
- Leung A.Y, and S. Foster. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics, 2nd ed. New York: John Wiley & Sons, Inc. 1996.
- Pappas SR. Institüt für Marktokologie. Nr. 22/183. Vagosca, Bosnia – Herzegovina 2003.
- Newall CA, Anderson LA, Phillipson J.D. Herbal Medicines. A Guide for Health-Care Professionals. London: The Pharmaceutical Press. 1996.
- Butani L, Afshinnik A, Johnson J, et al. Amelioration of tacrolimus-induced nephrotoxicity in rats using juniper oil. Transplantation 2003; 76(2): 306-311.
- Burits M, Asres K, Bucar F. The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus procera. PhytotherRes 2001; 15(2):103-8. PMID: 11268106 [PubMed - indexed for MEDLINE]
- Afanasev IB, Dorozhko AL, Brodoski AV, et al. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. BiochemPharmacol 1989; 38: 1763– 1769.
- Elmastas M, Gulcin I, Beydemir S, Kufreviogulu OI, et al. A Study on the In Vitro Antioxidant Activity of Juniper (Juniperus communis L.) Fruit Extracts. Analytical Letters 2006; 39: 47-65.
- Wiley–VCH Verlag GmbH, Weinheim, fed. Rep. of Germany. Lipid- Fett. Volum 101, issue 10, pages 395-400. Published online: 12 Oct 1999.
- Markkanen T. Antiherpetic agent from juniper tree (Juniperus communis), its purification, identification, and testing in primary human amnion cell cultures. Drugs Exp ClinRes 1981; 7: 691-697.
- Cosentino S, Barra A, Pisano B, et al.isi FM, Palmas F. Composition and antimicrobial properties of Sardinian Juniperus essential oils against foodborne pathogens and spoilage microorganisms. J FoodProt 2003; 66 (7): 1288-1291.
- Johnston WH, Karchesy JJ, Constantine GH, Craig AM. Antimicrobial activity of some Pacific Northwest woods against anaerobic bacteria and yeast. PhytotherRes 2001; 15 (7): 586-8. PMID: 11746838 [PubMed - indexed for MEDLINE]
- Filipowicz N, Kaminski M, Kurlenda J, Asztemborska M, Ochocka JR. Antibacterial and antifungal activity of juniper berry oil and its selected components. PhytotherRes 2003; 17(3): 227-231.
- Parker WA, Whisman BA, Apaliski SJ, Reid MJ. The relationships between late cutaneous responses and specific antibody responses with outcome of immunotherapy for seasonal allergic rhinitis. J Allergy ClinImmunol 1989; 84 (51): 667-77. PMID: 2809024 [PubMed - indexed for MEDLINE]
- Bayazit V. Cytotoxic effects of some animal and vegetable extracts and some chemicals on liver and colon carcinoma and myosarcoma. SaudiMed J 2004; 25 (2): 156-163.
- Wang WS, Li EW, Jia ZJ. Terpenes from Juniperus przewalskii and their antitumor activities. Pharmazie 2002; 57(5): 343-5. PMID: 12061261 [PubMedindexed for MEDLINE
- Gholam AN, Seddigheh A, Nizal SZ and Hamid S. Anti-oxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol CellBiochem 2003; 246 (1-2): 193-196.
- Wiley –VCH Verlag GmbH, Weinheim, fed.Rep. of Germany. Lipid- Fett. Volum 101, issue 10, pages 395- 400. Published online: 12 Oct 1999. 20. Witzum JL. The oxidation hypothesis of atherosclerosis. Lancet 1994;.344: 793-798.
- Witzum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogeneis. J. ClinInvest 1991; 88: 1785-1792.
- Lindgren FT, Jensen LC, Wills RD, Freeman NK. Flotation rates, molecular weights, and hydrated densities of the low density lipoproteins. Lipids 1969; 4: 337– 344.
- Pyevich D, Bogenschutz MP. Herbal diüretics and lithium toxicity [letter]. M. J. Psychiatry 2001; 158: 1329.
- Edwards PA, Ericsson J. Signaling molecules derived from the cholesterol biosynhetic pathway: mechanism of action and possible roles in human disease. Courr. Opin Lipidol. 1998; 9(5): 433-440.
- Cucchiara B, Scott EK. Use of statins in CNS disorders. J Neur Sci 2001: 187; 81-89.
- Heuer T, Gerards H, Pavw M. Toxic liver damage caused by HMG-CoA–redüktase inhibitor. MedClin 2000; 11: 642-644.
- Kayaalp O. Hypolipidemic drugs: Rational Treatment in Terms of Medical Pharmacology. 8rd ed. Hacettepe TAS Bookselling Ltd. Sti. Ankara, 2000; pp 567-587. 28. Dujovne CA. New lipid lowering drugs and new effects of old drugs. Curr Opin Lipidol 1997; 8: 362-368.
- Syrov VN, Abzalova MK, Khushbaktova ZA and Sultanov MB. Hypolipidemic and antiatherosclerotic properties of terpenoid coumarins. Institute of the Chemistry of Plant Substances, Academy of Sciences of the Uzbek SSR, Tashkent Received 1984.
- Tuzmen I, Hafizoglu H. Terpene groups in essential oils of Juniperus L. cones and leaves of grown in Turkey. ZKU Bartin Faculty of Forestry Journal Vol I-II. 2004; 6(6): 88-95.
- Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999; 286: 1946-1949.
- Sanchez de Medina F, Gamez MJ, Jimenez I, et al. Hypoglycemic activity of juniper "berries". Planta Medica 1994; 60(3): 197-200.
- Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia 1990; 33(8): 462-464.
- Avci G, Kupeli G, Eryavuz A, Yesilada E and Kucukkurt I, Antihypercholesterolaemic and. Antioxidant activity assessment of some plants used as remedy in Turkish folk medicine. JEthnopharm 2006; 107(3): 418-423.
- Baser KHC, Honda G, Miki W. Herb Drugs and Herbalists in Turkey. Tokyo Publishing & Printing Co. Ltd 1986.
- Williams E, Gokool N. Primary Care: An Update on the Role of Plant Sterols and Stanols in the Management of Hypercholesterolaemia. CNMag 2005; 5: 36- 38.