Research Article - Biomedical Research (2017) Volume 28, Issue 1
Anti-hepatitis B effect of lamivudine loaded HPMC microspheres in DHBV infected ducklings: optimization by 32 full factorial design
Wei Guo Li1, He Qun Wang1, Yunju Shi2*1Department of Infectious Disease, Zhumadian Center Hospital, Henan, PR China
2School of Nursing, Zhengzhou railway vocational and technical college, Zhengzhou, Henan, PR China
- *Corresponding Author:
- Yunju Shi
School of Nursing
Zhengzhou railway vocational and technical college
Zhengzhou, PR China
Accepted date: June 22, 2016
Abstract
The present investigation demonstrates the effect of polymer and surfactant concentration on encapsulation efficiency along with testing and comparing the antiviral activity of Lamivudine microsphere with existing dosage forms. The O/O solvent evaporation method was used to prepare system in which Hydroxy propyl cellulose (HPMC) was used as release retarding polymer while Span 80 serves the function of surfactant. The statistical data was obtained using 32 full factorial design by selecting Polymer concentration (A), surfactant concentration (B) and encapsulation efficiency (Y1) as independent variables and dependent variables respectively. The interaction between polymer and drug was studied using FTIR spectra while surface morphology and physical nature of was characterized by FESEM and XRD analysis, respectively. The encapsulation efficiency was found to be in the range of 74.40% (F3) to 84.80% (F5) whereas DR values of formulated batches showed wide variation from 67.83% to 91% which can be stated as effect of concentration of polymer i.e. HPMC. The optimized formulation when tested on DHBV infected duckling was found to be greater in its DHBV inhibitory action (49.75% in 72 hr) when compared to plain drug (up to 32 hrs) and SR tablet dosage form (up to 48 hrs). This study had successfully demonstrated Lamivudine loaded sustained release microspheres using HPMC as potential drug delivery system for the treatment of Hepatitis-B.
Keywords
Anti-viral activity, HBV, Hepatitis-B, Hydroxy propyl methyl cellulose, Lamivudine microsphere.
Introduction
Hepatitis B, the most common infectious disease in the world caused by HBV (hepatitis B virus) primarily targets liver with visibility of symptoms like gianotti-crosti syndrome, membranous glomerulonephritis [1-3] and identification is based on reoccurrence of symptoms like vomiting, nausea, mild fever, jaundice [4]. The transmitted of HBV occurs through blood or other body fluids, unprotected sexual contacts while infected needles being the most common route [5]. Today use of nucleoside analogues and immunomodulators are the two major preferred options for treatment but side effect of interferons limit their use. Therefore need for alternate strategies for drug delivery has emerged with increasing patient compliance [6].
Lamivudine is a nucleoside reverse transcriptase inhibitors used for the treatment of hepatitis B [7]. The marketed oral formulation of Lamivudine exhibits side effects in gastrointestinal tract (GIT) and central nervous system (CNS) [8]. Lamivudine has bioavailability between 80 to 87 % with half-life of 5 to 7 hr [9] which ultimately aids to reduce the dosing frequency and side effects of the Lamivudine. In patients with chronic hepatitis B and compensated liver disease, Lamivudine reduces serum HBV-DNA level which is undetectable upon 1-2 months of treatment. A 1 year treatment is associated with normalization of transaminase levels and substantial histological improvement in many patients. In patients with decompensate cirrhosis, Lamivudine has been administrated pre and post liver transplantation; it is well tolerated, suppresses serum HBV-DNA and appears effective in preventing HBV reinfections of the graft [10].
In attempt to overcome the drawbacks of conventional system, the micro-particulate drug delivery systems were innovated which encloses polymeric particles, microspheres. When compared to conventional drug solution or suspension the polymeric micro particles promises the controlled and prolonged drug delivery. These well-established systems help to prolong drug retention time, decreasing drug clearance from body tissue with improved therapeutics effect and patient compliance. The slow and steady drug delivery had proven to prolong the drug release when compared with rapid injections. This slow delivery can be achieved by coupling the active drug to micro particles. The use of micro particles to deliver a drug at the local site has been proposed. These Polymeric microspheres (1-1000 μm) are usually produced by solvent evaporation method by using either synthetic or natural materials. Among the various polymeric material hydroxypropyl methylcellulose (HPMC) is broadly used due to its solubility characteristics in gastrointestinal fluid and compatibility with wide range of formulation types [11]. The polymeric excipient HPMC is selected as release retardant as it controls the release rate based on different phenomenon including gelation. The popularity of the polymer may be the result of its nontoxic nature, ease of compression, and capability to accommodate and high drug loading. Based on the difference of molecular weight and viscosity, it is equipped with characteristics and application of emulsification, bonding, thickening, gelation and film forming. High viscosity grades can be used to control the release the rate from polymeric matrix whereas comparatively low viscosity grades channel molecule when used for sustained release preparations [12].
The present study deals with the effect of variables like surfactant and polymer on the microspheres (prepared using solvent evaporation method) with statistical application of 3 level factorial design. Lamivudine loaded micro particles are proposed to deliver drug for a prolonged period of time and compared to the marketed formulation for its release characteristic as well as antiviral activity. All preformulation characteristics were also evaluated.
Materials and Methods
Procurement of antiviral agent and other excipient
Lamivudine was obtained as gift sample from Shouguang Fukang Pharmacy Factory (Shandong, China). Span-80, Light liquid paraffin, methanol, Hydroxy Propyl Methylcellulose (HPMC), Potassium dihydrogen phosphate, sodium hydroxide purchased from MERCK (Germany). All other materials were of analytical or reagents grade.
Design of experiments
A variety of factors were evaluated in the formulation of Lamivudine micro particles using 32 full factorial designs [13]. The independent variables selected were the concentration of polymer (A, mg), concentration of surfactant (B, % v/v) varied at three levels (-1, 0, +1). The drug EE (Y1 %) was selected as dependent factors. The statistical experimental data were analysed using the Design-Expert® Software. Table 1 shows experimental design used to study the effects on the EE.
| Independent Variable | Low level (-1) | Medium level (0) | High level (+1) |
| A= Polymer conc (mg) | 100 | 200 | 300 |
| B= Surfactant conc (%) | 0.1 | 0.55 | 1 |
| Dependent Variables | |||
| Y1= % Encapsulation efficiency |
Table 1: Variable and three levels.
Lamivudine microsphere production
Lamivudine loaded microsphere formulations were prepared by using HPMC and span-80. Oil in oil solvent evaporation technique was employed for the preparation of microspheres. Briefly, accurately weighed quantities of HPMC and Lamivudine dissolved in sufficient quantity of methanol (Organic phase). Light liquid paraffin and span-80 were mixed as per the design (External phase).
The resulting organic phase was injected using a syringe with a 21.5 G needle into light liquid paraffin as an external phase containing varying concentrations of span 80. After 60 min, nhexane was added into the solution for hardening of the microspheres [14]. Microspheres were separated by centrifugation.
Particle size determination
Particle size was determined by Particle size analyser Malvern ZS 200. Micro particles were dispersed in distilled water. Using Particle size analyser 10 mg micro particles were observed, and average size was determined.
Drug encapsulation efficiency
About 100 mg of microspheres was taken and triturated with phosphate buffer pH 6.8 and transferred to 100 mL volumetric flask. The volume was made up to 100 mL and mixed well. The solution was then kept aside for 12 hours. It was sonicated in ultrasonicator and then filtered through membrane filter 0.45 μm and estimated for drug content by measuring the absorbance at 270 nm. The drug entrapment efficiency was calculated using the formula.
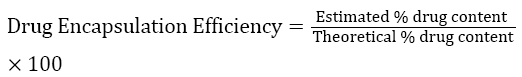
In-vitro dissolution study
The USP rotating-paddle dissolution rate apparatus (USP XXII type II apparatus) was used to study drug release from the 100 mg microspheres. The dissolution parameters were 37 ± 2ºC, 100 rpm; 900 ml of phosphate buffer pH 6.8 were maintained for all the formulations. About 5 ml of aliquot samples were withdrawn at specified intervals and after suitable dilution were assayed by using UV-Visible spectrophotometer at 270 nm.
Scanning electron microscopy
The morphology of microspheres was investigated by scanning electron microscopy (FESEM-S 4800, Hitachi, Japan) at a working distance of 8.6-8.8 mm and accelerating voltage of 1.0 kV. The particles were examined for shape, size and surface characteristics.
X ray diffraction study
Crystalline and amorphous nature of the Lamivudine, HPMC and Lamivudine loaded microspheres were analysed by X-ray diffraction. Powder X-ray diffractometer was carried out with X-ray diffractometer (Miniflex, Rikagu).
Anti-hepatitis activity of lamivudine
The anti-hepatitic activity of Lamivudine formulation was tested on one day old Ducklings as test animals. The animals were previously tested for their suitability for experiment under the guidelines of, Chinese Academy of Medical Sciences, under permit of the Ethical Committee for Animal Experiments of the Institute of Medicinal Biotechnology. The test animals were fed with proper diet of food and water. One day old Pekin ducklings were selected and inoculated with DHBV-positive serum via intravenous route. The optimized formulation of Lamivudine was administered via oral route after starting the treatment on 7th day after inoculation with dose of optimized formulation equivalent to 50 mg/kg of Lamivudine.
The serum DHBV-DNA levels in treated and control groups (3 animals in each group) were served as indicator for the activity. The animals were divided into the following groups:
• T0 = ducklings at initiation of the treatment
• T2= ducklings at 2th day of treatment
• T3=ducklings at 3rd day of treatment
• Control= Untreated animal
On the days T0, T1, T2, T3 blood samples (after preparing sera) were analysed for DHBV-DNA levels. The similar study was performed by replacing optimized Lamivudine formulation with plain Lamivudine, sustained release tablet of Lamivudine (marketed formulation) and results were compared on basis of % inhibition. ANOVA test was used for statistical analysis to find out the statistical significant results.
Detection of DHBV-DNA in serum of infected animals
The blot test assay was used to detect the DHBV-DNA from the serum of infected ducklings. On nitrocellulose paper, approximately 100 μl serum samples were spotted using micropipette. The filters (previously hybridized with full length α-32P labelled genomic DNA probe) after performing denaturation, neutralization and fixation (at 800ºc, 2 hr), were auto radiographed and quantitative measurement was done using following formula:
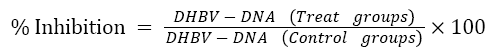
Where,
T0, T1, T2 = test groups
DHBV-DNA= concentration of DHBV-DNA in sera.
ANOVA test was used for statistical analysis to find out the statistical significant results.
Results
32> full-factorial experimental design is one of the most prominent statistical modelling techniques. This methodology includes the effect of independent variables at three levels (-1, 0, +1) which is widely useful for the development and optimization of the relationship between measured variables and a number of independent variables in the form of polynomial equations. This 32> full-factorial experimental design includes lesser experiments, simple design as compare to BoxBehnken, central composite and D-optimal design. A 32> full-factorial experimental design was used to evaluate the effect of independent variables on the dependent variables. During the formulation of Lamivudine sustained release micro particles, methanol was used as a solvent as both HPMC and Lamivudine were soluble in methanol. The Encapsulation efficiency was found to be in the range of 74.40 % (F3) to 84.80 % (F5). Table 2 represents the EE of all batches. F5 Formulation batch has highest EE (% 84.80).
| Batch No. | Polymer conc (mg) | Surfactant conc (%) | Encapsulation Efficiency (%) |
| F1 | 200 | 1.0 | 80.20 |
| F2 | 200 | 0.55 | 83.80 |
| F3 | 100 | 0.10 | 74.40 |
| F4 | 300 | 0.10 | 79.30 |
| F5 | 300 | 0.55 | 84.80 |
| F6 | 100 | 1.0 | 76.20 |
| F7 | 200 | 0.10 | 77.00 |
| F8 | 200 | 0.55 | 83.60 |
| F9 | 200 | 0.55 | 83.20 |
| F10 | 100 | 0.55 | 80.20 |
| F11 | 200 | 0.55 | 82.90 |
| F12 | 300 | 1.00 | 82.40 |
| F13 | 200 | 0.55 | 83.00 |
Table 2: % Encapsulation efficiency (Y1).
These values were compared with observed and predicted values with residuals for all the batches shown in Table 3. The model equation obtained for DL (Y1) was given by,
Y1= 83.26+2.62A+1.35B-0.67A2 -4.57B2 +0.33AB
| Batch No | Actual value | Predicted Value | Residual |
| F1 | 80.20 | 79.98 | 0.22 |
| F2 | 83.80 | 83.26 | 0.54 |
| F3 | 74.40 | 74.39 | 0.014 |
| F4 | 79.30 | 78.97 | 0.33 |
| F5 | 84.80 | 85.21 | -0.41 |
| F6 | 76.20 | 76.44 | -0.24 |
| F7 | 77.00 | 77.34 | -0.34 |
| F8 | 83.60 | 83.26 | 0.34 |
| F9 | 83.20 | 83.26 | -0.062 |
| F10 | 80.20 | 80.04 | 0.16 |
| F11 | 82.90 | 83.26 | -0.36 |
| F12 | 82.40 | 82.32 | 0.081 |
| F13 | 83.00 | 83.26 | -0.26 |
Table 3: Diagnostic case statistics of response variable (Y1).
A positive value in the above regression equation represents the synergistic effect of the independent variable while a negative value represents the antagonistic effect [15-17]. There is only a 0.01% chance that a "Model F-Value" this large could occur due to noise. Values of "Prob>F" less than 0.0500 indicates model terms are significant. In this case A, B, A2 and B2 are significant model terms. Values greater than 0.1000 indicate the model terms are not significant.
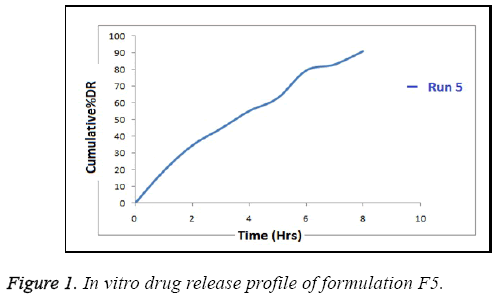
In vitro release profile of optimized formulation when analysed with respect to time was found sustained for run 5 as shown in Figure 1. It shows initial bursting providing for loading dose followed by maintained dose by sustained release of drug. The overall wide release patterns i.e. from 67.83-91% were seen for other batches.
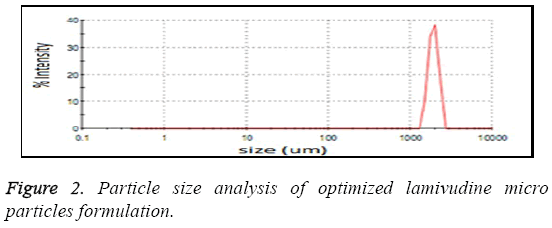
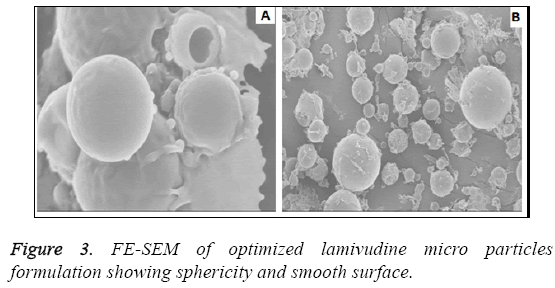
The particle size analysis revealed that all the particles are in micron size range. The average particle size was found to be in the range of 1.1-3.0 μm as shown in Figure 2. Most of the microspheres were found to be in the range of 1.1-3 μm. The size range analysis shows that about 85% of the particles were up to 2.75 μm. The size distribution was found normal in all batches. The mean size distribution was increased as the concentration of polymer in the batches increase. The surface morphology of lamivudine micro particles when scanned by SEM as shown in Figure 3, the surface of micro particles was found to be smooth and regular, devoid of any cracks. The micro particles were discrete and free flowing in nature.
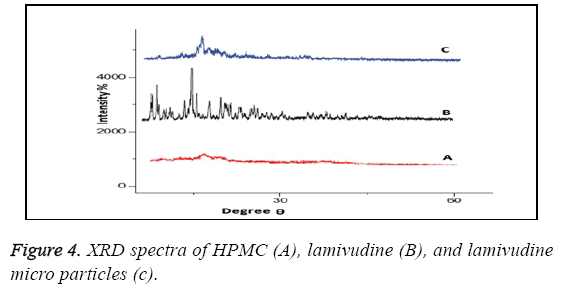
XRD patterns as shown in Figure 4 of pure lamivudine (B) showed sharp peaks at 2θ-scattered angles of 14, 22, 25, and 27 confirm the crystalline nature of drug. Intensities of drug peaks were also decreased in the formulation.
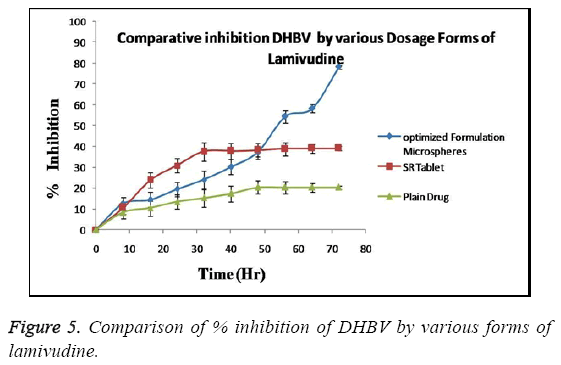
The anti-hepatitis (or antiviral) activity of optimized Lamivudine formulation was evaluated in DHBV infected duckling model. The activity when compared to Lamivudine SR tablet and pure Lamivudine the inhibitory efficiency of Lamivudine microspheres was found to be greater than that of other formulation as shown in Figure 5.
The optimized formulation of Lamivudine microspheres exhibit sustained increase in inhibition of DHBV- DNA in animal model up to 72 hrs when compare to pure drug (32 hr) and SR tablet (48 hr) with no remarkable increase in % inhibition thereafter (p<0.05). The formulation maintains significant (p<0.05) anti-HBV activity even in single dose administration, in contrast other formulations (which often require frequent administration). This long lasting protective effect can be explained by the release retarding nature of polymers used in the formulation [18].
Discussion
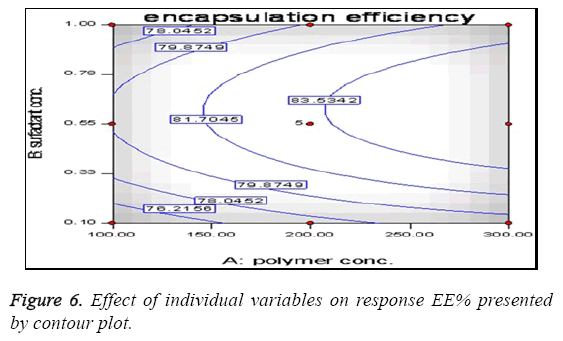
The present study was an attempt to formulate Lamivudine micro particulate drug delivery system using HPMC as polymer with aim to localize the drug at absorption site with increase BA, prolong and enhance the anti-hepatitis activity and overall patient compliance. The system was successfully formulated using solvent evaporation method and HPMC as polymer. Before proceeding for the formulation the preformulation study was done to check the effect of HPMC and span 80 on EE and DR to decide the concentration of polymer and surfactant. All the batches were prepared according to design expert software using different levels of drug and polymer and evaluated for various parameters like Encapsulation efficiency, particle size, surface morphology, In vitro release, Antiviral activity etc. The results of all above parameters are predicted. The effect of polymer concentration on %EE is shown in Table 2. It can be stated that polymer concentration has the significant effect on %EE which increases with increase in polymer concentration in consecutive formulations. This effect can be the result of property of polymer to embed dug in its matrix. The minor errors while performing experiments are liable to short deviations in experimental values. These differences are checked through use of software giving predicted values which can be compared with actual values for studying the error. Table 3 shows good correlation between actual and predicted value. This correlation can be further confirmed through statistical analysis as shown in Table 4. The value of correlation coefficient (R2) was found to be 0.9912, indicating good fit. The results from Table 5 showed that %EE affected by the independent variables like A, B, A2, B2 and AB. Among these 5 variables A (<0.0001), B (<0.0001) and B2 (<0.0001) had significant effect on the EE. The interaction term AB show how the EE changes when 2 variables are simultaneously changed. As shown in Table 5, the Model F-value of 158.07 implies the model is significant. There is only a 0.01% chance that the values obtain is due to some error and not the result of accurate experimentation. "Prob>F" less than 0.0500 indicates model terms are significant i.e. have effect on variables. In this case A, B, A2 and B2 are significant model terms. The effects of A and B with their interaction on the EE are shown in Figure 6 as a contour plot. In case of polymer concentration, also at 0 levels the EE was maximum. The EE was further decreased at +1 and -1 level. Therefore the rank order for EE with respect to polymer concentration was found in this order 0>-1>+1. In case of surfactant concentration, at 0th level the EE was maximum (84.80%). The EE was further decreased at +1 and -1 level and in these cases it was 76.20 % and 74.40%. Therefore the rank order for EE with respect to surfactant concentration was found in this order 0>-1>+1. The in vitro release pattern shows sustained release. This release pattern helps to maintain constant plasma profile with initial load of dose. Due to rate controlling polymer HPMC the drug release from system is controlled so as to provide for prolong duration of action. As the system provides for prolong duration of action the frequency of dosing is significantly decreased. The particle size of the microsphere obtained in the range of 1.1-3.0 μm. This uniform size distribution helps to maintain the flow characteristics of the formulation. The surface of micro particles was found to be smooth and regular, devoid of any cracks. This characteristic reduces the chances of drug to leach out from polymeric HPMC coat giving formulation better integrity. SEM also confirms the discrete and free flowing in nature of microspheres. This property establishes flow characteristics in micro particles providing ease in formulating further dosage forms. The XRD data shows three characteristic peaks of polymer (HPMC), Lamivudine and microspheres. The crystalline peaks observed in XRD pattern of pure drug were disappeared in the micro particle formulation, indicates amorphous nature of the drug after entrapment. From the Figure 5, one can observe that optimized formulation shows sustained and highest % inhibition up to 72 hrs while sustained release tablet of Lamivudine shows increase in inhibition up to 48 hrs only and plain drug inhibits for 32 hrs only. After this time period both the formulations show no increase in inhibition and become stagnant. The increase in inhibition can be stated as the result of prolong and sustained release of drug from the formulation which gives more margin for drug to act locally. These comparisons seems to be sufficient to prove superiority of optimized microsphere formulation over conventional dosage forms in % inhibition of DBHV for prolong period of time. HPMC is well known polymer for its compatibility with large type of dosage forms aids in sustaining the release of drug from the formulation. The results also show the effect of HPMC on the drug release pattern from microspheres. Hence, the results clearly indicate superiority of Lamivudine microsphere formulation over conventional dosage (pure drug as well as SR tablet) in inhibiting DBHV in experimental duckling model.
| Models | R2 | Adjusted R2 | Predicted R2 | Std. Deviation | Press | Remarks |
| Response Y1 | ||||||
| Linear | 0.4004 | 0.2805 | -0.1839 | 2.79 | 153.81 | ……… |
| 2FI | 0.4037 | 0.2049 | -1.5504 | 2.93 | 333.31 | ……… |
| Quadratic | 0.9912 | 0.985 | 0.9522 | 0.4 | 6.2 | Suggested |
| Cubic | 0.995 | 0.988 | 0.9466 | 0.36 | 6.94 | ……… |
Table 4: Summary of results of regression analysis for response Y1.
| Source | DF | Sum of squares | Mean square | F value | P value |
| Model for Y1 | 9 | 128.77 | 25.75 | 158.07 | <0.0001 |
| A | 1 | 41.08 | 41.08 | 252.15 | <0.0001 |
| B | 1 | 10.93 | 10.93 | 67.12 | <0.0001 |
| A2 | 1 | 1.23 | 1.23 | 7.55 | 0.0286 |
| B2 | 1 | 57.61 | 57.61 | 353.61 | <0.0001 |
| AB | 1 | 0.42 | 0.42 | 2.59 | 0.1514 |
Table 5: ANOVA model for y1.
Conclusion
In the aforementioned study successful attempt was made to formulate the micropaticulate system of Lamivudine using HPMC as polymer with enhanced and prolong anti hepatitis activity and improved patient compliance. From careful study performed we can conclude that lamivudine microspheres can be prepared successfully using HPMC as polymer. Formulation no. 5 shows the highest % EE and excellent In-vitro sustained drug release. Particle size analysis shows that particles were in the range of 1.1-3 μm with smooth and no cracky surface, an ideal property of microspheres. The anti DHBV activity study shows enhanced and prolong anti hepatitis activity over pure drug and SR tablet providing for high therapeutic efficiency. Statistical analysis confirms that above observations are not the parts of error but are result of careful and accurate experimentation.
Conflict of Interest
The authors declare that they have no financial or other conflict of interests in relation to this research and its publication.
References
- Norio O, Kyuichi F, Shingo T, Minoru N, Takafumi I, Hitoshi A. Novel Patterns of Amino Acid Mutations in the Hepatitis B Virus Polymerase in Association With Resistance to Lamivudine Therapy in Japanese Patients With Chronic Hepatitis B. J Med Vir1999; 59: 270-276.
- Eugene RS. Lamivudine for Hepatitis B in Clinical Practice. J Med Vir 2000; 61: 386 -391.
- Trepo C, Guillevin L. Polyarteritisnodosa and extrahepatic manifestations of HBV infection: the case against autoimmune intervention in pathogenesis.J Autoim2001;16: 269-274.
- Terrault N, Roche B, Samuel D. Management of the hepatitis B virus in the liver transplantation setting. European American perspLivTranspl2005; 11: 716-732.
- Hepatitis-B, FAQs for the PublicTransmission. U.SCenters for Disease Control and Prevention (CDC). Retrieved2011: 11-29.
- Juthamas R, Sorada K, Siriporn D. The development of injectable gelatin/silk fibroin microspheres for the dual delivery of curcumin and piperine. J Mater Sci Mater Med 2014; 25: 401-410
- Ola AM, Ashmoony MM, Tamer E. Spray dried lamivudine microspheres. J Pharm Res and Opi2014; 4: 1-7.
- Betty JD. Human immunodeficiency virus (HIV)-antiretroviral therapy. Drug Dis Manag 2000; 1555-1582.
- Sweetman SC. Martindale-The Complete Drug Reference, 35edn. London. Pharmaceutical Press London 2007.
- Condreay LD,Willems GP, Layrargues. Lamivudine treatment for decompensated cirrhosis resulting from chronic hepatitis B.Healthcoat 2000; 31: 207-210.
- Huichao W, Shouying LY. The application of biomedical polymer material hydroxyl propyl methyl cellulose (HPMC) in pharmaceutical preparation.J chem pharm Res 2001; 6: 155-160.
- Kajal G, Subrata C, Arunabha Nanda. Hydroxypropyl methylcellulose in drug delivery. Der Pharmacia Sinica2011; 2:152-168.
- Carla V, Filomena A, António J. The size of solid lipid nanoparticles: An interpretation from experimental design. Colloid Surface B 2011; 84: 117-130
- Jincheng W, Xingyu D, Sihao C. Microencapsulation of Capsaicin by Solvent Evaporation Method and Thermal Stability Study of Microcapsules. Lou Colloid J 2013; 75: 26-33.
- Sinha B, Muller H, Moschwitzer J. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size.Int J Pharm 2013; 453: 126-141.
- Nayak BS, Nayak UK. Lamivudine loaded microspheres for oral use: design, development and Establishment of in vivo-in vitro correlation. Asian JPharmClin Res 2009; 2:101-109.
- Nasir V, Sadhana R. Study on formulation variables of methotrexate loaded mesoporous MCM-41 nanoparticles for dissolution enhancement. Eur J Pharm Sci 2012; 45: 8-18
- Sood S, Jain K, Gowthamarajan K. Optimization of curcuminnano emulsion for intranasal delivery usingdesign of experiment and its toxicity assessment. CollSurfac B 2014; 113: 330-337.