Research Article - Biomedical Research (2016) Volume 27, Issue 4
Anti-Helicobacter pylori activity of phytochemicals from Brassica rapa L.
Ah-Young Kim1,2#, Mi-Ran Ki1#, Se-Hoon Park3, Young-Tae Ahn3, Eun-Mi Lee1,2, Eun-Joo Lee1,2, Se-Il Park4, Chul-Sung Huh3*, Kyu-Shik Jeong1,2*
1Department of Pathology, College of Veterinary Medicine, Kyungpook National University, Daegu, Republic of Korea
2Stem Cell Therapeutic Research Institute, Kyungpook National University, Daegu, Republic of Korea
3Korea Yakult Co., Ltd., Yongin, Gyeonggi-do, Republic of Korea
4Cardiovascular Product Evaluation Center, College of Medicine, Yonsei University, Seoul, Republic of Korea
#These authors contributed equally as first authors
- *Corresponding Authors:
- Kyu-Shik Jeong
Department of Pathology
Kyungpook National University
Republic of Korea - Chul-Sung Huh
Korea Yakult Co., Ltd.
Yongin
Gyeonggi-do
Republic of Korea
Accepted date: March 30, 2016
Abstract
Helicobacter pylori (H. pylori) is a major causative agent of atrophic gastritis and peptic ulcer as well as associated with gastric carcinogenesis. The bacteria not only can persistently colonize the stomach by evading the immune system of the host, but also has antibacterial drug resistance. Thus, the need for alternative medicine to effectively eradicate the bacteria has become more important. Phytochemicals from crucifer vegetables are well-known alternative medicines as antibacterial substances. Among various crucifer vegetables, a turnip (Brassica rapa L.), in the family Brassicaceae, was evaluated in the present study because of its popularity on ethnopharmacology and the advantage of long-term storage after harvest. The evaluation was done by colony formation unit calculation, urease activity test, anti-H. pylori IgG titer measurement, proinflammatory cytokine measurement, and histopathological assessment after sacrificing the infected mouse model. Among the experimental groups, the group treated with a high dose of myrosinase-reacted turnip roots exhibited a distinctive anti-H. pylori activities, such as reduced bacterial colonization and increased anti-H. pylori IgG titer. Thus, the present study suggests a turnip as a new functional dietary regimen for the treatment of H. pylori infection. Furthermore, the present study can be utilized as a basis for the development of safe and effective drugs for other infectious alimentary diseases.
Keywords
Antimicrobial, Glucosinolates, Helicobacter pylori, Phytochemicals, Turnip.
Introduction
Helicobacter pylori (H. pylori) is a gram-negative, microaerophilic helical bacillus which inhabits various areas of the stomach and duodenum [1] and is closely related to chronic active type B gastritis, peptic ulcers, gastric cancer, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma [2]. More than half of the world’s population harbors the bacteria in the upper gastrointestinal tract [1].
In addition, most infections by this organism are acquired during childhood and persist during one’s lifetime unless eradicated properly [3]. Commonly used triple therapy which combines two antibiotics and a proton pump inhibitor (PPI) or subsequently developed quadruple therapy which combines two antibiotics, PPI, and bismuth are still the most effective first-line treatments [4].
However, the eradication failure rate still remains at 10-40% [5] because of drug resistance. Besides, side effects from the antibiotics and poor patient compliance have urged many researchers to find novel regimens against H. pylori infection by utilizing antimicrobial peptides, small molecule inhibitors and naturopathic substances. Among those three areas, naturopathic agents can be subcategorized into probiotics and phytochemicals [6].
To date, various medicinal plant extracts have been validated as promising treatments for H. pylori infection with their antibacterial activity or anti-inflammatory activity [7]. Herein, we focused on a turnip (Brassica rapa L.), which has been widely researched for its nutritional benefits and pharmacological effects on various diseases [8], but not assessed for anti-H. pylori effectivity.
The aim of the present study was, therefore, to evaluate the anti-H. pylori activity of phytochemicals from turnips to develop effective dietary cures for H. pylori infection.
Materials and Methods
Animals
Six week-old, female, specific pathogen free C57BL/6 mice (n=42) weighing 15-18 g were used in this study. Mice that were free of Helicobacter spp. were initially obtained from Orient Bio Inc. and housed in a mouse holding isolator during the entire experimental period. Mice were given food and water ad libitum. Animal experiments were performed in accordance with NIH guidelines for the care and use of laboratory animals and with the approval from the institutional animal care and use committee of Kyungpook National University (KNU 2010-86). Mice were divided into 6 groups of similar mean body weights. The six groups were as follows: uninfected control (CON), negative control (NC), positive control (PC), experimental group 1 (G1), experimental group 2 (G2) and experimental group 3 (G3).
Bacteria
Sydney strain (SS1), a mouse-adapted strain of Helicobacter pylori, was used [9]. The bacteria was grown on blood agar plates containing 5% defibrinated horse blood for 2 days at 37°C under microaerophilic (10% CO2) conditions for subculturing. Within 24 hours from being subcultured onto new plates, the bacteria were harvested, resuspended in Muller- Hinton broth, and injected into each group of mice at 1109 colony-forming units/mouse except for the CON (uninfected control) group by oral gavage. The CON group received H. pylori-free Muller-Hinton broth instead of H. pylori culture. Inoculation was done 3 times per week at 2-day intervals.
Preparation of Brassica rapa L. powder
In this study, we used Brassica rapa L. which was cultivated on Kanghwa island, Korea during autumn. The root of Brassica rapa L. was washed with water and cut into appropriate sizes. After adding the same weight of water, the Brassica rapa L. was ground in a blender. Ground Brassica rapa L. was freeze-dried and the resulting powder was named HY2. Additionally, another ground Brassica rapa L. was adjusted to pH 7 and L-ascorbic acid (1 mm), a cofactor for myrosinase, was added to the solution. After incubation for 3 hours at 37 to carry out the myrosinase reaction, the solution was filtered with a 50 μm mesh screen. The filtrate was freezedried and the resulting powder was named HY3. Both HY2 and HY3 were extracted from fresh turnip roots at a 0.333% (10 g of freeze-dried powder per 3 Kg of turnip roots) yield.
Experimental design
All mice were given a normal rodent diet for the first week of the experiment. From the next day after the completion of three H. pylori inoculations to the endpoint of the experiment, the mice received different diets for 4 weeks according to their group as follows. The CON and NC received normal rodent diets; The PC received amoxicillin (20 mg/Kg/day) containing diets; The G1 received HY2 (200 mg/Kg/day) containing diets; The G2 and G3 received HY3 (100 mg/Kg/day and 200 mg/Kg/day, respectively) containing diets. Each group diet was prepared by mixing a corresponding sample with normal rodent diet powder taking into consideration the mean daily food intake of a mouse and the dosage of the samples. Edible paste was used to make the diets into pellet form again. Thus, the diet for the CON and NC groups was the remade pellet of the pulverized normal rodent diet.
Assessment of H. pylori in infected mice
Four weeks after H. pylori infection, the mice were killed and the stomachs were removed and washed with saline to remove food scraps. The stomach was divided longitudinally into two parts. One-half of the stomach was fixed in 10% formaldehyde and other-half was minced for the recovery of H. pylori from the stomach.
The culture of stomach tissue: The quantitative colonization of H. pylori SS1 was evaluated by culturing stomach tissue homogenates on selective agar plates as previously described [10]. Horse blood agar plates supplemented with DENT (Oxoid, UK) were used for the selective growth of H. pylori [11]. After plating serial 10-fold dilutions of stomach tissue homogenates on the selective agar plates, they were incubated at 37°C under microaerophilic (10% CO2) conditions for 4 days. Colony forming units per gram of tissue (CFU/g) was calculated from the counted bacterial colonies.
Immunohistochemistry and histopathology: The second method was the immunohistochemical detection of H. pylori infection in gastric biopsies. A stomach sample from each mouse was fixed in 10% neutralized buffered formalin for routine processing and embedded in paraffin. The sections were cut to a 4 thickness and stained by immunohistochemistry (IHC) with polyclonal rabbit antibodies of H. pylori (Dako, Denmark). The number of H. pylori, which were colonized in gastric pits, was also counted in five random fields per slide at 1000x magnification.
Histopathology
For histopathological analysis, formalin-fixed paraffin-embedded sections of the stomach were stained with hematoxylin and eosin (H&E). Because eosinophils are known as a prominent component of H. pylori-induced gastritis [12], the number of eosinophils that infiltrated the gastric mucosa was counted in five random fields per slide at 400x magnification.
Measurement of urease activity
To detect the urease activity of colonized H. pylori into stomach tissue, all infected mice of each group were analyzed. Minced stomach tissue was mixed with same volume of urease activity test solution: 1 M urea and 10 μl of phenol red pH indicator (Sigma-aldrich, Germany) in 1ml of distilled water. After vortexing, tissue was reacted for six hours with the test solution and centrifuged at 4,000 rpm for 10min in 4. Supernatant from each sample was isolated and given to read the absorbance at 595 nm.
Measurement of anti-H. pylori IgG level
To detect the level of serum anti-H. pylori IgG antibodies, Enzyme-Linked Immunosorbent Assay (ELISA) was used as described in Goo et al. [13].
Serum inflammation markers measurements
Serum interleukin-17 (IL-17), Tumor Necrosis Factor-alpha (TNF-α) and Keratinocyte Chemoattractant (KC) were measured using multiplex detection kits and analyzed with a Bio-Plex Suspension array system (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All values are presented as the mean ± SE except for the cytokine assay which was done as the median ± SE. Statistical analyses were determined using one-way analysis of the variance (ANOVA) followed by Duncan’s post hoc test for multiple comparisons. The value of statistical significance was set at p<0.05. All statistical analyses were performed with the SPSS 18.0 software program (IBM, USA).
Results
Effect on H. pylori colonization
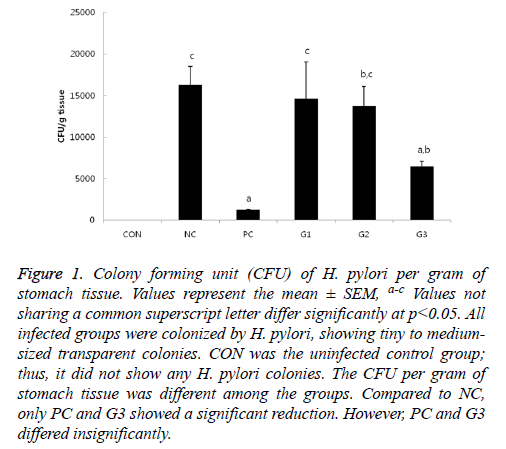
Except for CON, H. pylori colonization was observed in all infected mice groups by quantitative culture with tiny to medium-sized transparent colonies on H. pylori selective blood agar plates. As expected, the NC and PC group had the highest and lowest CFU, respectively (Figure 1).
Figure 1: Colony forming unit (CFU) of H. pylori per gram of stomach tissue. Values represent the mean ± SEM, a-c Values not sharing a common superscript letter differ significantly at p<0.05. All infected groups were colonized by H. pylori, showing tiny to mediumsized transparent colonies. CON was the uninfected control group; thus, it did not show any H. pylori colonies. The CFU per gram of stomach tissue was different among the groups. Compared to NC, only PC and G3 showed a significant reduction. However, PC and G3 differed insignificantly.
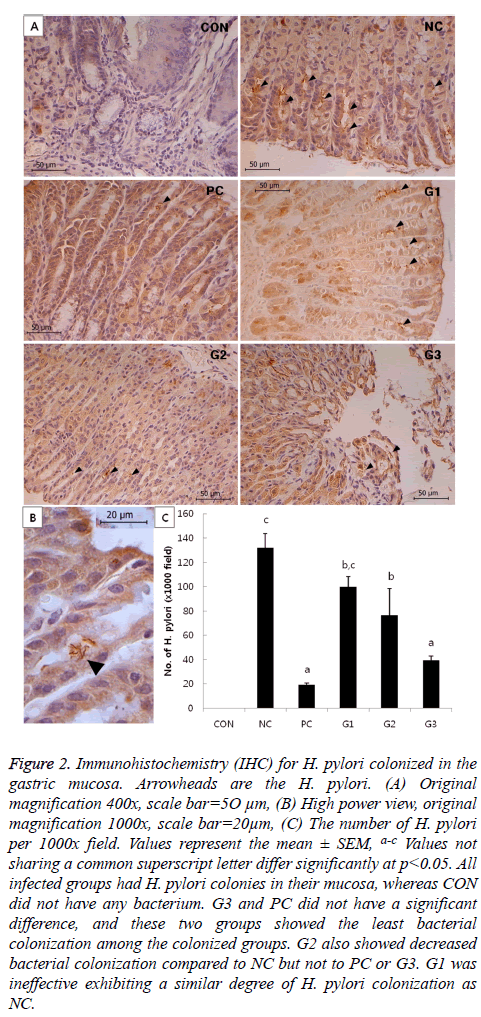
There were no significant differences in bacterial colonization among the G1, G2, and NC groups. However, the CFU of G3 was significantly lower compared to that of NC, which was comparable to PC in anti-H. pylori effect. In addition, bacterial colonization of H. pylori was also examined by immunohistochemistry (IHC). The results of IHC (Figure 2) had a similar pattern as the results in Figure 1.
Figure 2: Immunohistochemistry (IHC) for H. pylori colonized in the gastric mucosa. Arrowheads are the H. pylori. (A) Original magnification 400x, scale bar=5O μm, (B) High power view, original magnification 1000x, scale bar=20μm, (C) The number of H. pylori per 1000x field. Values represent the mean ± SEM, a-c Values not sharing a common superscript letter differ significantly at p<0.05. All infected groups had H. pylori colonies in their mucosa, whereas CON did not have any bacterium. G3 and PC did not have a significant difference, and these two groups showed the least bacterial colonization among the colonized groups. G2 also showed decreased bacterial colonization compared to NC but not to PC or G3. G1 was ineffective exhibiting a similar degree of H. pylori colonization as NC.
Effect on gastric mucosal histology
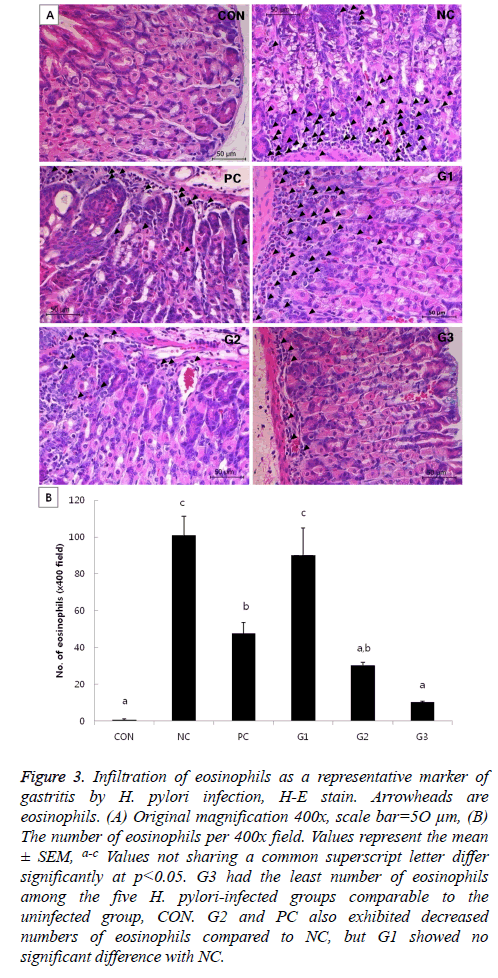
In the H&E-stained slides, the degree of gastritis was briefly assessed by counting the number of eosinophils per view. Though CON is the uninfected control group, mice in this group had a few eosinophils under normal status. G3 seemed to have more eosinophils than CON, but the result was not significant. Although PC and G2 had significantly more eosinophils than CON, the numbers of eosinophils in those groups were significantly less than that in the NC group. Meanwhile, G1 had a similar degree of eosinophil infiltration as the NC group (Figure 3).
Figure 3: Infiltration of eosinophils as a representative marker of gastritis by H. pylori infection, H-E stain. Arrowheads are eosinophils. (A) Original magnification 400x, scale bar=5O μm, (B) The number of eosinophils per 400x field. Values represent the mean ± SEM, a-c Values not sharing a common superscript letter differ significantly at p<0.05. G3 had the least number of eosinophils among the five H. pylori-infected groups comparable to the uninfected group, CON. G2 and PC also exhibited decreased numbers of eosinophils compared to NC, but G1 showed no significant difference with NC.
Urease activity
H. pylori secrets urease to protect itself from the acidic environment of the stomach as neutralizing gastric acids by ammonia production [14].
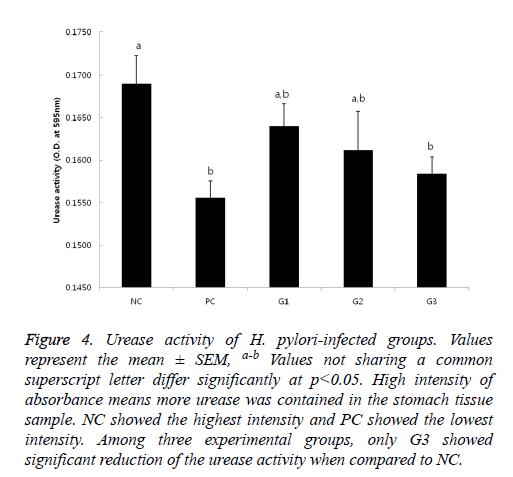
If urease exists, the color of the test solution gets red because the enzyme hydrolyzes urea, produces ammonia and makes the pH increase. Thus, it means that the higher absorbance is shown, the more urease from presumably more or active H. pylori exists. The results of urease test (Figure 4) also showed a similar pattern as the results in Figure 1. The weakest activity was detected on PC group and the strongest activity was seen in NC group; Only in G3, significant reduction of urease activity was observed.
Figure 4: Urease activity of H. pylori-infected groups. Values represent the mean ± SEM, a-b Values not sharing a common superscript letter differ significantly at p<0.05. High intensity of absorbance means more urease was contained in the stomach tissue sample. NC showed the highest intensity and PC showed the lowest intensity. Among three experimental groups, only G3 showed significant reduction of the urease activity when compared to NC.
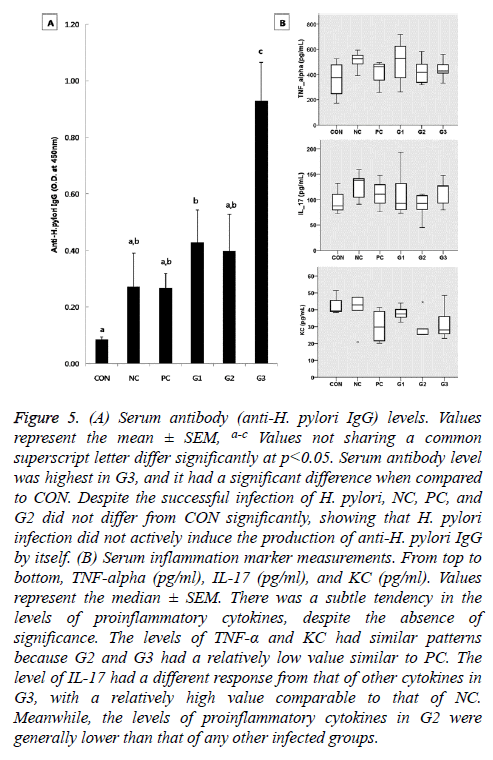
Anti- H. pylori IgG
The serum antibody level was significantly raised in G1 and G3 compared to CON. Especially, G3 exhibited the highest level of anti-H. pylori IgG among the five infected groups. Despite the successful infection of H. pylori, the level of anti- H. pylori antibodies in NC, PC, and G1 did not differ significantly from that of CON (Figure 5A).
Figure 5: (A) Serum antibody (anti-H. pylori IgG) levels. Values represent the mean ± SEM, a-c Values not sharing a common superscript letter differ significantly at p<0.05. Serum antibody level was highest in G3, and it had a significant difference when compared to CON. Despite the successful infection of H. pylori, NC, PC, and G2 did not differ from CON significantly, showing that H. pylori infection did not actively induce the production of anti-H. pylori IgG by itself. (B) Serum inflammation marker measurements. From top to bottom, TNF-alpha (pg/ml), IL-17 (pg/ml), and KC (pg/ml). Values represent the median ± SEM. There was a subtle tendency in the levels of proinflammatory cytokines, despite the absence of significance. The levels of TNF-α and KC had similar patterns because G2 and G3 had a relatively low value similar to PC. The level of IL-17 had a different response from that of other cytokines in G3, with a relatively high value comparable to that of NC. Meanwhile, the levels of proinflammatory cytokines in G2 were generally lower than that of any other infected groups.
Effect on proinflammatory cytokines production
Although there were no significant differences in the levels of proinflammatory cytokines among the groups, those of TNF-α and KC had similar patterns as shown by the CFU of the H. pylori infected groups according to the diet received (Figure 5B). The levels of proinflammatory cytokines in G2 were generally lower than that of any other infected groups. IL-17 had a different response from the other cytokines in G3 with a high IL-17 level comparable to that of the NC group (Figure 5B).
Discussion
Throughout the overall assessments, G3 had a similar degree of anti-H. pylori activity as the PC group, which was treated with amoxicillin, and thus, was significantly different from the rest of the experimental groups. Each test ingredient contained in the diets of the G1, G2 and G3 group was from Brassica rapa L., but the ingredients were structurally different according to their different preparation methods, and that could cause significant differences in their anti-H. pylori activity. Even though myrosinase exhibits considerable activity in the absence of vitamin C, its activity is potentiated by ascorbate [15] because ascorbate is a cofactor for myrosinase and acts as a substitute for the catalytic base [15].
It is already known that the antibacterial effect of broccoli is due to sulforaphane, made by myrosinase, not to glucoraphanin, the precursor of sulforaphane [16]. Thus, the phenomenon that substances from the same plant, Brassica rapa L., showed significantly different potencies depending on the enzymatic reaction could be due to the same reason as in the case of broccoli. The difference was not due to vitamin C itself, because vitamin C is heat labile and the experimental diets were passed through a drying machine when they were made. Thus, myrosinase activation could be a crucial step in producing antibiotic substances from Brassica rapa L. Meanwhile, the G2 and G3 group received the same substance with different doses. Considering the results of the experiment, it seemed that HY3 was as effective as amoxicillin (20 mg/Kg taken a day) in anti-H. pylori activity when taken at 200 mg/Kg a day.
Even though the exact effective substance is unidentified, it was known that Brassica rapa L. contains various glucosinolates such as gluconapoleiferin, gluconapin, glucobrassicanapin, gluconasturtiin etc [17]. Thus it was assumed that the effective substances could be a kind of isothiocyanate converted from the glucosinolates of Brassica rapa L. by myrosinase. In addition to the antibiotic function against H. pylori, a higher dose of HY3 augmented anti-H. pylori IgG production and alleviated gastritis characterized by infiltration of eosinophils in the gastric mucosa. HY3 tended to decrease the levels of proinflammatory cytokines including TNF-α and KC, though not significant, which was comparable to that of amoxicillin. Because KC is a murine homolog of human IL-8, this tendency was consistent with that other anti- H. pylori phytochemiclas in previous studies lower the IL-8 secretion in H. pylori-infected human gastic epithelial cells [18,19].
However, a higher dose of HY3 had a mild tendency to increase the level of IL-17, which was contrary to the results by from amoxicillin and a lower dose of the HY3. According to Karbir [20], IL-17 has a dual role in infection and vaccination of H. pylori. In infection, T regulatory cells (Tregs) suppress the inflammatory reaction driven by IL-17, causing bacterial persistence. Thus, it might be assumed that the Th17/ IL-17 pathway could have a role in mucosal host defense against H. pylori. However, Shi et al. [21] revealed that H.pylori burden and inflammation were both reduced when IL-17 activity was blocked in vivo or when IL17-/- mice were used. Thus, it is rather reasonable to think that the Th17/IL-17 pathway plays a pathogenic role in H.pylori infection by promoting mucosal inflammation and contributing to bacterial colonization [21]. Contrary to this, the early events in the immune response of immunized and challenged mice include the recruitment of Th17 cells and the production of IL-17 which triggers gastric inflammation and then overcomes the Treg responses leading to bacterial clearance.
According to previous studies, both Th17 and Th1 subsets are absolutely required for vaccine-induced protection [22], and persistence of H. pylori is mediated by an ineffective adaptive immune response that is characterized by insufficient Th1 and Th17 responses and inappropriate Tregs activation [12]. Seemingly G3 had a high IL-17 level comparable to that of the NC group, but the underlying mechanisms could be different between the two groups. Generally, H. pylori infection induces the Th1-dominated immune reaction in both humans and C57BL/6 mice [23]. However, it was recently revealed that Th1 cell responses are modulated by the preceding Th17 responses. That is, the high IL-17 level expressed in the NC group could be attributed to the detrimentally biased immune reaction by H. pylori infection. However, it was estimated that the elevated level of IL-17 in G3 was because of the positively potentiated immune response, considering that the G3 group had the highest level of blood anti-H. pylori IgG and the lowest level of gastric mucosal inflammation. Thus, it was deduced that the dosage of HY3 for G3 induced a sufficient immune response to clear the bacteria as in the case of the immunized mice.
According to a nutritional genomics study [24], it seems certain that brassicaceous vegetables are profitable for cancer prevention, but it is hard to predict the individual contribution of each ingredient of a broccoli because there are various components affecting various cell-signaling pathways that jointly contribute to protection against cancer development. Likewise, various ingredients from a turnip may jointly evoke protective function against H. pylori by affecting various pathways. Though broccoli is a representative brassicaceous plant for H. pylori treatment [6], it is a flower vegetable which has short storage period after harvest. In the other hand, turnip root can be stored for a long time in a refrigerator without spoilage, because it is a root vegetable. Therefore, a turnip is more convenient to regularly ingest and to experience the long-term effect of medication than other stem or flower vegetables.
In conclusion, Brassica rapa L. can help to eradicate H. pylori when persistently taking an adequate amount of phytochemicals derived from it because turnip extract not only suppressed both H. pylori colonization and inflammatory cells infiltration, but also boost immune response against the bacteria. Treatment of H. pylori needs long-term medication even if it is dealt with antibiotics. However, turnip roots can be plan B instead of antibiotics or other expensive and perishable medicinal plants for long-term treatment of H. pylori. Furthermore, our report also can be applied to develop new drugs to target several infectious alimentary diseases.
Acknowledgements
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI15C0001) and in part supported by Korea Yakult Co., Ltd.
Conflict of Interest
All authors declare that there is no conflict of interest.
References
- Njume C, Afolayan AJ, Ndip RN. Preliminary phytochemical screening and in vitro anti-Helicobacter pylori activity of acetone and aqueous extracts of the stem bark of Sclerocarya birrea (Anacardiaceae). Arch Med Res 2011; 42: 252-257.
- Yang JC, Shun CT, Chien CT, Wang TH. Effective prevention and treatment of Helicobacter pylori infection using a combination of catechins and sialic acid in AGS cells and BALB/c mice. J Nutr 2008; 138: 2084-2090.
- Moon JK, Kim JR, Ahn YJ, Shibamoto T. Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli (Brassica oleracea L.) sprouts. J Agric Food Chem 2010; 58: 6672-6677.
- Vitor JM, Vale FF. Alternative therapies for Helicobacter pylori: probiotics and phytomedicine. FEMS Immunol Med Microbiol 2011; 63: 153-164.
- Njume C, Jide AA, Ndip RN. Aqueous and Organic Solvent-Extracts of Selected South African Medicinal Plants Possess Antimicrobial Activity against Drug-Resistant Strains of Helicobacter pylori: Inhibitory and Bactericidal Potential. Int J Mol Sci 2011; 12: 5652-5665.
- Makobongo MO, Gilbreath JJ, Merrell DS. Nontraditional therapies to treat Helicobacter pylori infection. J Microbiol 2014; 52: 259-272.
- Zaidi SF, Muhammad JS, Usmanghani K, Sugiyama T. Review: Pharmacological ins and outs of medicinal plants against Helicobacter pylori: A review. Pak J Pharm Sci 2015; 28: 1171-1176.
- Jeong J, Park H, Hyun H, Kim J, Kim H, Oh HI, Hwang HS, Kim DK, Kim HH. Effects of Glucosinolates from Turnip (Brassica rapa L.) Root on Bone Formation by Human Osteoblast-Like MG-63 Cells and in Normal Young Rats. Phytother Res 2015; 29: 902-909.
- Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 1997; 112: 1386-1397.
- Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) 2009; 2: 353-360.
- Dent JC, McNulty CA. Evaluation of a new selective medium for Campylobacter pylori. Eur J Clin Microbiol Infect Dis 1988; 7: 555-558.
- Nagy TA, Allen SS, Wroblewski LE, Flaherty DK, Slaughter JC, Perez-Perez G, Israel DA, Peek RM, Jr. Helicobacter pylori induction of eosinophil migration is mediated by the cag pathogenicity island via microbial-epithelial interactions. Am J Pathol 2011; 178: 1448-1452.
- Goo MJ, Ki MR, Lee HR, Yang HJ, Yuan DW, Hong IH, Park JK, Hong KS, Han JY, Hwang OK, Kim DH, Do SH, Cohn RD, Jeong KS. Helicobacter pylori promotes hepatic fibrosis in the animal model. Lab Invest 2009; 89: 1291-1303.
- Paulo L, Oleastro M, Gallardo E, Queiroz JA, Domingues F. Anti-Helicobacter pylori and urease inhibitory activities of resveratrol and red wine. Food Research International 2011; 44: 964-969.
- Burmeister WP, Cottaz S, Rollin P, Vasella A, Henrissat B. High resolution X-ray crystallography shows that ascorbate is a cofactor for myrosinase and substitutes for the function of the catalytic base. J Biol Chem 2000; 275: 39385-39393.
- James D, Devaraj S, Bellur P, Lakkanna S, Vicini J, Boddupalli S. Novel concepts of broccoli sulforaphanes and disease: induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli. Nutr Rev 2012; 70: 654-665.
- Li S, Schonhof I, Krumbein A, Li L, Stutzel H, Schreiner M. Glucosinolate concentration in turnip (Brassica rapa ssp. rapifera L.) roots as affected by nitrogen and sulfur supply. J Agric Food Chem 2007; 55: 8452-8457.
- Rao YK, Lien HM, Lin YH, Hsu YM, Yeh CT, Chen CC, Lai CH, Tzeng YM. Antibacterial activities of Anisomeles indica constituents and their inhibition effect on Helicobacter pylori-induced inflammation in human gastric epithelial cells. Food Chemistry 2012; 132: 780-787.
- Zaidi SF, Muhammad JS, Shahryar S, Usmanghani K, Gilani AH, Jafri W, Sugiyama T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J Ethnopharmacol 2012; 141: 403-410.
- Kabir S. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter 2011; 16: 1-8.
- Shi Y, Liu XF, Zhuang Y, Zhang JY, Liu T, Yin Z, Wu C, Mao XH, Jia KR, Wang FJ, Guo H, Flavell RA, Zhao Z, Liu KY, Xiao B, Guo Y, Zhang WJ, Zhou WY, Guo G, Zou QM. Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J Immunol 2010; 184: 5121-5129.
- Hitzler I, Kohler E, Engler DB, Yazgan AS, Muller A. The role of Th cell subsets in the control of Helicobacter infections and in T cell-driven gastric immunopathology. Front Immunol 2012; 3: 142.
- Algood HM, Cover TL. Helicobacter pylori persistence: an overview of interactions between H. pylori and host immune defenses. Clin Microbiol Rev 2006; 19: 597-613.
- Ferguson LR, Schlothauer RC. The potential role of nutritional genomics tools in validating high health foods for cancer control: broccoli as example. Mol Nutr Food Res 2012; 56: 126-146.