Research Article - Journal of Food Technology and Preservation (2017) Volume 1, Issue 1
Antifungal and antiochratoxigenic properties of chemical preservatives in/of bread
Carla Luciana Gerez1*, Ana Yanina Bustos2, Graciela Font de Valdez31Center for Lactobacilli (Cerela Conicet), Chacabuco 145 (4000) Tucumán, Argentina.
2Center of Research and Transfer of Santiago del Estero (citse -conicet), Argentina.
3Faculty of Biochemistry, Chemistry and Pharmacy, Center for Lactobacilli (Cerela Conicet), National University of Tucumán, Tucumán, Argentina.
- *Corresponding Author:
- Carla Luciana Gerez
Bio-Chemistry
Centro De Referencia Para Lactobacilos, Tucumán
Argentina
Tel: +54-381 431-0465
Fax: +54-381 400-5600
E-mail: clugerez@cerela.org.ar
Accepted date: November 10, 2016
Citation: Gerez CL, Bustos AY, de Valedz GF. Antifungal and Antiochratoxigenic Properties of Chemical Preservatives In/of Bread. J Food Technol Pres. 2016;1:6-10
Abstract
The objective of this work was to study the efficiency of preservatives on growth and ochratoxin a (ota) production by Aspergillus niger 13 d at pH values often found in bakery products. the fungal growth inhibition was concentration and pH dependent. differences between calcium propionate (Cp) and potassium sorbate (ks) were found, observing that ks was less effective than Cp. no fungal growth was observed with 0.4% (w/w) of Cp at pH 6.0, while that the maximum concentration of ks (0.2%, w/w) was not able to inhibit 100% of fungal growth independentlyof pH evaluated. This study also demonstrates the influence of preservatives on ota production. ota was not detected when the growth was 100% inhibited, i.e., at pH 6.0 and 0.4% (w/w) of Cp. the concentration of Cp could be reduced at 0.3% (w/w) when the pH was lowered to 5.5 without risk of contamination with ota. In presence of ks, a moderate depletion of ota production was observed, however, the maximum concentration (0.2%, w/w) did not completely inhibit ota production. in addition, no stimulating effects on growth or ota levels were observed in any condition assayed. These “in vitro” studies must be corroborated by “in situ” conditions in bakery products and other Aspergillus fungus.
Keywords
Ochratoxin a; Aspergillus niger; Propionic acid; Sorbic acid.
Practical Applications
Ochratoxin a, a toxin produced by some species of Aspergillus, is one of the most abundant and the most toxic member of food-contaminating mycotoxins. It is noted that there is limited information concerning the effect of food-grade preservatives on the ota production by Aspergillus fungus. Our results corroborate that reduction of fungal growth is the most efficient way to prevent the formation of ota and reduce the human consumption of the toxin. On the other hand, our findings suggest that the effectiveness of preservatives is dependent on the strain as well as the concentration and pH values. Thus, the concentration of preservatives could be reduced when the pH was reduced, with no risk of ota production.
Introduction
Fungal contamination of foods and the subsequent risk of mycotoxins production represent significant economic losses and constitute a serious public health concern [1]. In fact, ochratoxin a (ota) produced by filamentous fungi, manly by Aspergillus (a.) and penicillium (p.) strains, is among the most important mycotoxins and is the most toxic member of the ochratoxins group [2]. Teratogenic, Embryotoxic, Genotoxic, Neurotoxic, Immunosuppressive, carcinogenic (iarc group 2b), and, NEP Hrotoxic effects produced by ota were demonstrated [3-5]. Contamination of wheat flour and bread with high levels of ota has long been reported [6,7]. In addition, bread making process only moderately destroyed the toxin [8]. The possibility of intake polluted bread is high, especially in developing countries. On the other hand, the use of mouldy bread to feed animals destined to human consume is a common practice to decrease economic losses [9]. Reducing the fungal growth and consequently preventing the formation of ota are the most efficient way to reduce the human consumption of the toxin. traditionally, many preservatives have been used to prevent fungal growth in food, being the organic acids the most used due to their status of gras (generally recognized as safe) by the American food and drug administration (fda) [10,11]. Salts of propionic and sorbic acids i.e. calcium propionate (Cp) and potassium sorbate (ks), are preservatives commonly added to prevent spoilage and insure food safety in bakery products among otherS [12]. These preservatives are highly effective against mold and bacteria, possess no health hazard,and bring little flavorat normal use rates [13] however, nowadays, consumers demand a reduction in the use of such preservatives. in addition, there are organic acids, such as sorbic acid, which have a certain acceptable daily intake (adi). in these cases, it is important to carefully select the required concentrations to reduce the fungal growth since previous studies have suggested that the use of suboptimal concentrations of a preservatives could stimulate growth of spoilage fungi or/and mycotoxin production in penicillum, fusarium, and Aspergillus strains [14-16]. It is well known that dissociated active form of the organic acids is the responsible of their antimicrobial effect. Nevertheless, to our knowledge, very little information is available demonstrating the effect of pH variation at different concentrations of preservatives on the antifungal activity and inhibition of ota production by Aspergillus strains. In this sense, a recent study showed that the sensitivity of Aspergillus species against acetic and sorbic acids was dependent on the strain as well as the pH and acids tested [10]. Remarkably, under-dosing of sorbic acid stimulated the production of ochratoxin an in isolates of a. carbon Arius and A. niger. Therefore, the aim of this work was to evaluate the efficacy of Cp and ks on control of growth and ota production by A. niger 13 d at different pH values, representing conditions that commonly occur in bakery products.
Materials and Methods
Microorganisms
The Ochratoxigenic A. niger 13 d belongs to the culture collection of national university of Río Cuarto (Unrc), Córdoba, Argentina. This strain was stored at -20°C in 400 g l-1 glycerol and grown in malt extract agar (mea; difco laboratories, usa) plates at 25°C for 7 days. The conidia were collected with sterile soft agar (tween 80, 0.5 g l-1 and agar, 1.0 g l-1), quantified using a Haemocytometer.
Growth inhibition: In vitro assays
The effect of Cp and ks on A. niger 13 d was evaluated used plates containing wheat bread medium (wbm). the wbm agar medium was prepared as follows:2% (w/v) wheat flour 000 type (71.1% carbohydrates, 2.8% fiber, and10% protein),2% (w/v) agar (Britania Laboratory, Argentina) supplemented with different concentrations selected according argentine alimentary code for packaged sliced breads: Cp (from 0.1% to 0.4%w/w) or ks (from 0.05 to 0.2%,w/w). The pH was adjusted to 5.0, 5.5 and 6.0 by addition of hcl (cicarrelli laboratory, argetina) and checked using a pH-meter (Sartorius Agpt-10 Model, Goettingen, Germany). The plates were inoculated with the spore suspension of A. niger 13d by central puncture. Plates containing wbm agar at different pH values (5.0, 5.5 or 6.0) without the addition of preservatives were inoculated (105conidia/ml) with A. niger 13 d and used as control. Inoculated plates were placed on sealed polyethylene bags and incubated at 30°C for 10 days. The radial extension rates were determined according to Mitchell et al. [17].
Extraction and analysis of ota
The extraction of ota was done after 10 days incubation at 30°C according to gerez, et al. [18]. Three agar plugs were removed from the mycelium growth and extraction was done with methanol. The mixture was centrifuged (14.000 g, 10 min), evaporated to dryness and stored at 5°C. The ota concentration was determined using elisa assay (r1311 ridascreen®ochratoxin a kit, 30/15-r-biop Harm ag, germany) according to manufacturer’s instructions.
Statistical Analysis
The Statistical analysis was carried out with the Statistica 5.5 program (Statsoft, Tulsa, Ok, Usa). Results of three independent assays are presented as mean values ± standard deviation (sd).
Results
Effect of preservatives on A. niger 13d growth
Table 1 shows the effect of different concentration of Cp and ks on growth rates of A. niger 13d at three pH values. The growth of A. niger 13d was not affected by the pH tested (in absence of preservatives) and a fungal growth rate of 1.39cmd-1 was reached in all assays after 10 days. However, when preservatives were incorporated, the inhibition was dependent on the ks and Cp concentration as well as the pH values. In fact, increase in the amount of Cp was necessary to inhibit the growth of the A. niger 13d with increasing pH values. No fungal growth was observed with 0.3% (w/w) of Cp at pH 5.0 and 5.5; however, at pH 6.0 was necessary increase the concentration to 0.4% (w/w) to achieve similar results. On the contrary, ks was not able to complete inhibits fungal growth at any concentrations and pH values tested. The highest concentration allowed by the argentine alimentary code for packaged sliced breads (0.2%, w/w) only reduced 64% of growth rate independently the pH of the medium.
Table 1. Effect of preservatives and Ph on Aspergillus niger 13d growth.
* Fungal growth rate: Cm of colony days -1.
| Preservatives | Concentration (%) | Ph Values | Fungal Growth Rate* | Maximum Mycelial Growth¥ |
|---|---|---|---|---|
| 5.0 | 1.39 ± 0.01a | 8.4± 0.1 A | ||
| 0 | 5.5 | 1.39 ± 0.01a | 8.5 ± 0.1 A | |
| 6.0 | 1.39 ± 0.01a | 8.5 ± 0.0 A | ||
| Calcium Propionate | 5.0 | 0.68 ± 0.04 A | 5.9 ± 0.2 A | |
| 0.1 | 5.5 | 0.71 ± 0.04 A | 5.9 ± 0.2 A | |
| 6.0 | 0.89 ± 0.02 B | 7.1 ± 0.3 B | ||
| 5.0 | 0.25 ± 0.06a | 0.7 ± 0.1 A | ||
| 0.2 | 5.5 | 0.35 ± 0.05 B | 2.7 ± 0.4 B | |
| 6.0 | 0.42 ± 0.01 B | 2.8 ± 0.4 B | ||
| 5.0 | 0.00 ± 0.00 A | 0.0 ± 0.0 A | ||
| 0.3 | 5.5 | 0.00 ± 0.00 A | 0.0 ± 0.0 A | |
| 6.0 | 0.28 ± 0.06 B | 0.8 ± 0.0 A | ||
| 5 | 0.00 ± 0.00 A | 0.0 ± 0.0 A | ||
| 0.4 | 5.5 | 0.00 ± 0.00 A | 0.0 ± 0.0 A | |
| 6 | 0.00 ± 0.00 A | 0.0 ± 0.0 A | ||
| Potassium Sorbate | 0.05 | 5.0 | 0.89 ± 0.01 A | 8.5 ± 0.1 A |
| 5.5 | 1.03 ± 0.15 A | 8.5 ± 0.1 A | ||
| 6.0 | 0.91 ± 0.09 A | 8.5 ± 0.1 A | ||
| 0.1 | 5.0 | 0.56 ± 0.04 A | 4.5 ± 0.3 A | |
| 5.5 | 0.59 ± 0.08 A | 5.1 ± 0.5 A | ||
| 6.0 | 0.63 ± 0.08 A | 5.8 ± 0.0 A | ||
| 0.2 | 5.0 | 0.54 ± 0.12 A | 3.3 ± 0.7 A | |
| 5.5 | 0.45 ± 0.03 A | 3.7 ± 0.3 A | ||
| 6.0 | 0.50 ± 0.07 A | 4.4 ± 0.2 A |
¥Fungal colony diameter (Cm) in WBM agar medium with or without preservatives after 10 days at 30°C.
V
variables with the same superscript letter in the same column show no significant differences between them (P<0.05).
Effect of preservatives on ota production
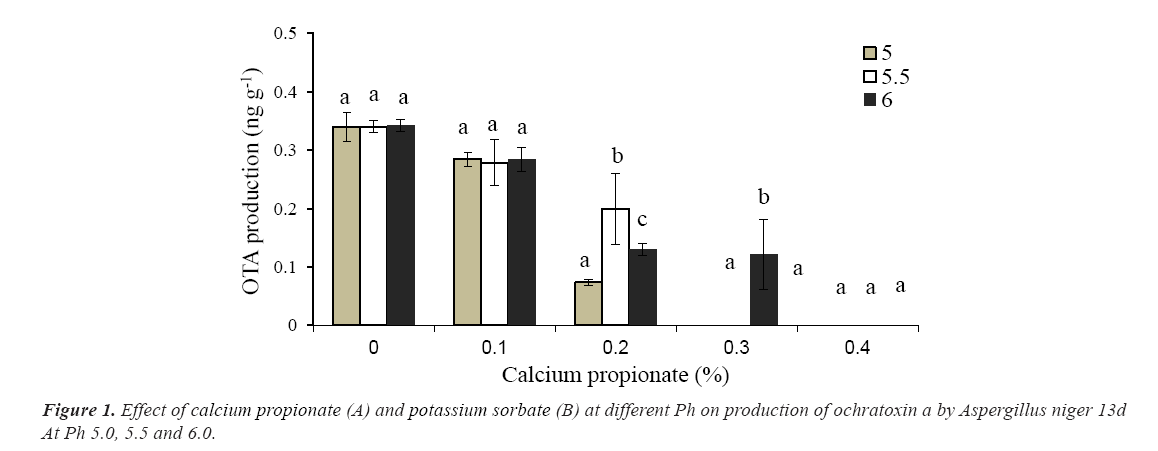
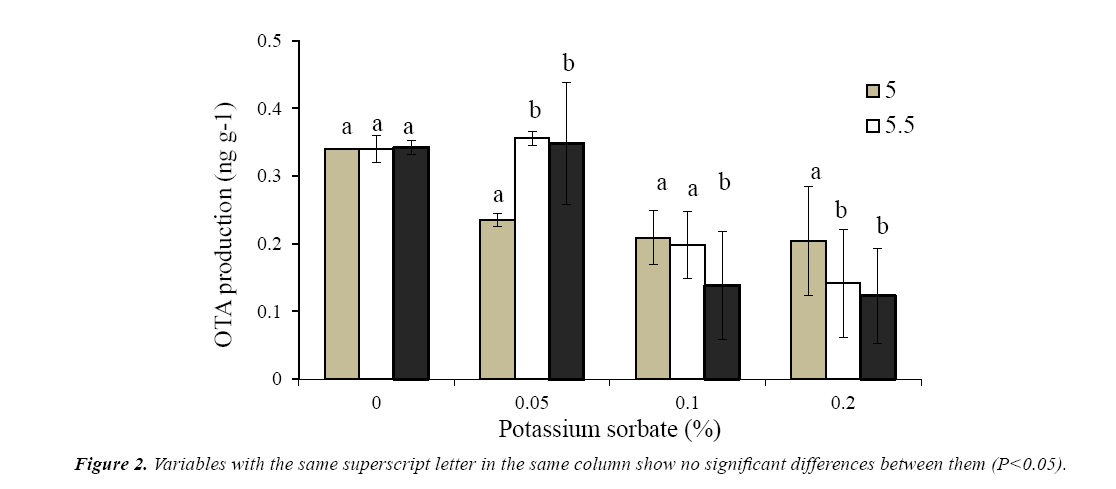
The production of ota by A niger 13d after 10 days incubation in wbm agar medium is presented in Figure 1. The maximum concentration (0.342 ng g-1) was achieved in the absence of preservatives regardless the pH of the medium. As was observed in growth experiments, Cp was the most effective preservative to inhibit ota production. In presence of the highest concentration of Cp, a strong depletion in ota levels was detected. No ota production with 0.3% (w/w) at pH 5.0 and 5.5of Cp was observed; however at pH 6 only a reduction of 65% was reached. In presence of ks, a moderate depletion of ota production was observed with the higher concentration and pH tested, while with 0.05% (w/w) of ks at pH 5.5-6.0, no effects on ota levels were observed. The highest concentration tested of ks (0.2%, w/w) only reduced from 40 to 64% the production of ota depending of the pH of the medium (Figure 2).
Discussion
In bakery processing, the most common type of microbial spoilage is mould growth and in many cases it is the major factor governing shelf life, representing significant economic losses. In addition, health hazards associated with the presence of myco toxins are also of concern. Acid organics and their derivate salts are the main preservatives used for preventing fungal spoilage of packed bread. However, in recent years, this approach has come under increased criticism since customers demand more natural foods (i.e. smaller amount of preservatives [19]. This study evaluates the effect of Cp and ks in combination with different pH values on growth and ota production by A. niger 13 d in order to understand how buffering situations that commonly occur in bread could affect their antifungal activity. Fungal growth inhibition was concentration dependent (Table 1). Tong and Draughon [20] reported similar results on a sulp Hureus nrrl 4077. However, other authors observed that suboptimal concentrations of preservatives stimulated growth of Aspergillus spp., p. verrucosum strains, and eurotium species [9,13,14]. Remarkably, a direct relationship between pH values and concentration of Cp able to inhibit the growth of A. niger 13d was observed. The effectively of organic acids is linked to the lipophilicity of the undissociated active form. This dissociated form can easily pass across the cell membrane and then accumulate within the cytoplasm, thereby causing loss of viability and cell destruction [21]. In this work, fungal growth was completely inhibited by 0.3% (w/w) of Cp at propionate at pH 5.0-5.5, but not at pH 6 (Table 1). At pH 6.0 was necessary to increase the concentration of propionate (0.4%, w/w) to inhibit 100% of fungal growth. these results could be explain due to that only 6% of the propionic acid is un dissociated at pH 6, compared to 44.93% at pH 5.0 (Table 2). similar results were observed with ks, however, this compound was less deleterious than Cp. in fact, the maximum concentration of ks (0.2%, w/w) allowed by the argentine alimentary code for packaged sliced breads was not able to inhibit 100% of fungal growth independently of pH evaluated. differences in inhibitory effects could be partially explained considering that ks has lower pka respect to Cp. thus, the un dissociated fraction active of ks is less to the Cp, e.g., 3.0 and 6.0%, respectively at pH 6.0. In this sense, membré et al. [19] reported no difference between Cp and ks, both applied at 0.2% (w/w) on p. Brevicompactum. On the contrary, some studies reported the ks as the most effective preservative to inhibit fungal contaminant species of bakery products [10,15,22] showed that sorbic acid has greater antifungal power than acetic acid for different aspergilli strains, since inhibitory concentrations of acetic acid was 30 to 50 times higher than concentrations of sorbic acid used to exert the same effect under the same conditions. The authors concluded that sorbic acid have direct effect on the cell membrane besides their cytoplasmic acidification action [23]. Differences in fungus strains and media composition are also important in testing the efficacy of preservatives. In addition, the differences in sensitivity observed among the moulds species may be related to their capacity to change the cell metabolism in answer to acid stress conditions [24]. In this work, the preservatives also had a significant effect on ota production. Similar to the effects observed for growth, ota production by A niger 13d was not affected by pH levels evaluated (in absence of preservatives). In this sense, [25] reported that the effect of pH on ota production by a. ochraceus and p. verrucosumwas dependent upon the fungi strain. On the whole, the influence of preservatives on ota production showed differences according to preservative studied. No ota was detected in treatments that led to 100% grow inhibition, i.e., with Cp at pH 5 and 5.5 and 0.3% (w/w). Similar results were showed [10,14,15], however, contrary to those reported by these authors, no stimulation of ota production was observed at any condition tested. These results provide useful information of the potential risk of ota accumulation in food matrices when using low concentrations of preservatives. the conclusion drawn from this work is that Cp (0.4% w/w, maximum concentration allowed by argentine alimentary code) is useful at controlling 100% of growth and ota production of A. niger 13 d. this concentration could be reduced at 0.3% (w/w) (maximum concentration allowed by European union) if the pH is lowered to 5.5. At present, studies are being conducted to evaluate the effect of preservatives on other ochrat oxigenic Aspergillus fungus and using bakery product.
Table 2. APercentage of the undissociated weak organic acid, calculated with the Henderson-Hasselbach equation [Ph=Pka + Log ([A]/[Ha])] where, Pka is the dissociation constant of the acid; [A] Is the concentration of molecules of the dissociated acid and [Ha] corresponds to the total concentration of undissociated acid. Variables with the same superscript letter in the same column show no significant differences between them (P<0.05).
| Percentage Of Undissociated Organic Acid Related To The Ph A | ||||
|---|---|---|---|---|
| Weak Organic Acid | Pka | Undissociated Weak Organic Acid (%) | ||
| Ph 5.0 | Ph 5.5 | Ph 6.0 | ||
| Propionic Acid | 4.88 | 44.93 | 26.13 | 6.05 |
| Sorbic Acid | 4.76 | 40.79 | 21.99 | 3.19 |
Acknowledgements
The authors acknowledge the financial support of conceit (pip 512) and anpcyt (pict 393).
References
- Hussein HS, Brasel JM. Toxicity Metabolism And Impact Of Mycotoxins On Humans And Animals Toxicology. 2001;167:101-34.
- Bento J, Pena A, Lino C, et al. Determination Of Ochratoxin A Content In Wheat Bread Samples Collected From The Algarve And Bragança Regions Portugal: Winter 2007 Microchemical Journal. 2009;91:165-69.
- Felizardo RJ, Câmara NO. Hepatocellular Carcinoma And Food Contamination: Aflatoxins And Ochratoxin A As Great Prompter. World Journal Of Gastroenterology: WJG. 2013;
- Meeting JF, WECOFA Organization WH & Safety IPOC, Safety Evaluation Of Certain Mycotoxins In Food: Food & Agriculture Org
- Woo CS, El-Nezami H. Maternal-Fetal Cancer Risk Assessment Of Ochratoxin A During Pregnancy Toxins. 2016;Vol-8, pp: 87
- Duarte S, Bento J, Pena A, et al. Ochratoxin A Exposure Assessment Of The Inhabitants Of Lisbon During Winter 2007/2008 Through Bread And Urine Analysis. Food Additives And Contaminants. 2009;26:1411-420.
- Paíga P, Morais S, Oliva-Teles T, et al. Determination Of Ochratoxin A In Bread: Evaluation Of Microwave-Assisted Extraction Using An Orthogonal Composite Design Coupled With Response Surface Methodology. Food and Bioprocess Technology. 2013;6:2466-477.
- Milani J, Heidari S. Stability Of Ochratoxin A During Bread Making Process. Journal Of Food Safety 2016.
- Perši N, Pleadin J, Kovačević D, et al. Ochratoxin A in Raw Materials and Cooked Meat Products Made From Ota-Treated Pigs. Meat Science. 2014;96:203-10.
- Alcano M, Jahn D, Scherer C. Susceptibility of Aspergillus Spp To Acetic And Sorbic Acids Based On Ph and Effect Of Sub-Inhibitory Doses of Sorbic Acid On Ochratoxin. A Production Food Research International. 2016;81:25-30.
- Ricke S. Perspectives On The Use Of Organic Acids And Short Chain Fatty Acids As Antimicrobials. Poultry Science. 2003;82:632-39.
- Samson RA, Hoekstra ES, Frisvad JC. Introduction To Food-And Airborne Fungi: Centraalbureau Voor Schimmelcultures CBS
- Alam S, Shah H, Afzal M, et al. Influence Of Calcium Propionate Water Activity And Storage Time On Mold Incidence and Aflatoxins Production in Broiler Starter Feed. Animal Feed Science and Technology. 2014;188:137-44.
- Arroyo M, Aldred D, Magan N. Environmental Factors And Weak Organic Acid Interactions Have Differential Effects On Control Of Growth And Ochratoxin. A Production By Penicillium Verrucosum Isolates In Bread, International Journal Of Food Microbiology. 2005;98:223-31.
- Marın S, Guynot M, Neira P, et al. Risk Assessment Of The Use Of Sub-Optimal Levels Of Weak-Acid Preservatives In The Control Of Mould Growth On Bakery Products. International Journal Of Food Microbiology. 2002;79:203-11.
- Schmidt-Heydt M, Baxter E, Geisen R, et al. Physiological Relationship Between Food Preservatives Environmental Factors Ochratoxin and Otapkspv Gene Expression by Penicillium Verrucosum. International Journal Of Food Microbiology. 2007;119:277-83.
- Mitchell D, Parra R, Aldred D, et al. Water And Temperature Relations Of Growth And Ochratoxin: A Production By Aspergillus Carbonarius Strains From Grapes In Europe And Israel. Journal Of Applied Microbiology. 2004;97:439-45.
- Gerez CL, Dallagnol A, Ponsone L, et al. Ochratoxin A Production By Aspergillus Niger: Effect Of Water Activity And A Biopreserver Formulated With Lactobacillus. Plantarum Crl 778 Food Control. 2014;45:115-19.
- Membré JM, Kubaczka M, Chèné C. Growth Rate And Growth–No-Growth Interface Of Penicillium Brevicompactum As Functions Of Ph And Preservative. Acids Food Microbiology. 2001;18:531-38.
- Tong CH, Draughon FA. Inhibition By Antimicrobial Food Additives Of Ochratoxin A Production By Aspergillus Sulphureus And Penicillium Viridicatum Applied And Environmental Microbiology. 1985;49:1407-411.
- Torino M, Taranto M, Sesma F, et al. Heterofermentative Pattern and Exopolysaccharide Production By Lactobacillus Helveticus Atcc 15807 In Response To Environmental Ph. Journal Of Applied Microbiology. 2001;91:846-52.
- Stratford M, Plumridge A, Archer DB, et al. Inhibition Of Spoilage Mould Conidia By Acetic Acid and Sorbic Acid Involves Different Modes Of Action Requiring Modification Of The Classical Weak-Acid Theory. International Journal Of Food Microbiology. 2009;136:37-43.
- Stratford M, Nebe-VC, Steels G, et al. Weak-Acid Preservatives: Ph And Proton Movements In The Yeast Saccharomyces Cerevisiae. International Journal Of Food Microbiology. 2013;161:164-71.
- Yang Y, Bastos M, Chen KY Effects Of Osmotic Stress And Growth Stage On Cellular Ph And Polyphosphate Metabolism In Neurospora Crassa As Studied By 31 P Nuclear Magnetic Resonance Spectroscopy Biochimica Et Biophysica Acta Bba-Molecular Cell Research. 1993;1179:141-47.
- Patterson M, Damoglou A. The Effect Of Water Activity and Ph On The Production Of Mycotoxins By Fungi. Growing On A Bread Analogue Letters In Applied Microbiology. 1986;3:123-25.