Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2022) Volume 12, Issue 91
Antidiarrheal and immunomodulatory activities of probiotic lactobacillus species isolated from palm wine, fowl gut and rotten banana fruit.
Chinedu J Ezeibe1, Malachy C Ugwu1*, Nonye T Ujam1,2, David C Nwobodo1, Chiamaka B Ugwu1, Anthony A Attama1,3
1Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka Anambra, Nigeria
2Department of Pharmaceutical Microbiology & Biotechnology, Enugu State University of Science and Technology, Enugu, Nigeria
3Department of Pharmaceutics, Faculty of Pharmaceutical Sciences University of Nigeria, Nsukka, Enugu, Nigeria
- Corresponding Author:
- Malachy C Ugwu
Faculty of Pharmaceutical Sciences
Nnamdi Azikiwe University
Awka Anambra, Nigeria
E-mail: mc.ugwu@unizik.edu.ng
Received: 25-June-2022, Manuscript No. AABPS-22-67679; Editor assigned: 28-June-2022, PreQC No. AABPS-22-67679(PQ); Reviewed: 13-July-2022, QC No. AABPS-22-67679; Revised: 16-July-2022, Manuscript No. AABPS-22-67679(R); Published: 23-July-2022, DOI:10.35841/2249-622X.91.131
Citation:Ugwu CM, Ezeibe JC, Ujam TN et al. Antidiarrheal and immunomodulatory activities of probiotic lactobacillus species isolated from palm wine, fowl gut and rotten banana fruit. Asian J Biomed Pharmaceut Sci. 2022;12(91):131
Abstract
This study characterized and evaluated the antidiarrhoeal and immunomodulatory effects of probiotic isolates from palm wine, rotten banana fruit, and fowl gut. The probiotic isolates were identified using Gram staining, colonial description, and biochemical reactions. The isolated probiotics were characterized by cultural characteristics on MRS agar and resistance to low pH. The probiotics were screened for anti-diarrheal and immunomodulatory activities. The gastrointestinal motility was also evaluated. Lactobacillus species were isolated from palm wine, fowl gut and rotten banana fruit sources. The antidiarrheal activity was time dependent. Percentage reduction of the mean weight of wet stool by fowl gut, palm wine, banana isolates and the standard drug were 23%, 39%, 36% and 67% respectively. Mean delay of onset diarrhea by distilled water, fowl gut, palm wine, banana isolates and loperamide were 63, 80, 95, 122, and 157 respectively. The test isolates caused a significant reduction in the distance traveled by charcoal meal. Percentage reduction by atropine, palm wine, banana, fowl gut isolates and distilled water are 32%, 40%, 53%, 66%, 93% respectively. The probiotic isolates possess anti motility, anti-secretory and immuno stimulatory properties and could be alternative to standard drugs for the management of diarrhea and prevention of the onset of infections.
Keywords
Probiotics, Palm wine, Banana, Fowl gut, Antidiarrheal activity, Immunomodulatory activity, Gastro intestinal motility.
Introduction
Probiotics are live microorganisms which when administered in adequate amounts provides health benefits on the host [1]. These organisms which constitute members of the commensal microorganisms are relevant for human and animal health, participating in several important biological functions. Such beneficial effects may be mediated by modulating immune responses particularly through balance control of proinflammatory and anti-inflammatory cytokines [2]. Most commonly formulated as fermentation products, probiotics counter pathogenic bacteria, support barrier function, and contribute to the regulation of the innate and adaptive immune responses [3-4].
As a natural and safer option, probiotics have emerged as new tools for the management and control of different diseases. The gastrointestinal tract of human, at different sites is inhabited by beneficial bacteria. These beneficial bacteria have a symbiotic relationship with the host, and are called probiotics. Probiotics (as derived from Latin and Greek words) means ‘‘for life’’. is defined in many ways. The commonest definition of probiotics is “live micro-organisms administered in adequate amounts which confer a beneficial physiological effect on the host”. Joint FAO/WHO experts’ consultation report defined probiotics as: live microorganisms which when administered in adequate amounts confer a health benefit on the host [1,3]. There are different types of probiotic bacteria, however, the commonly reported groups of bacteria include Lactobacilli and Bifido bacteria [4,5]. Also, among the probiotic strains, the Lactobacillus and Bifido bacterium exhibit a great ability to survive gastric transit and, thus, are extensively used in many Pharmaceutical and dairy probiotic products [6].
In recent times, probiotics have been consumed to inhibit pathogens and increase shelf life of foods. Probiotics promote a healthy balance of gut bacteria and have been linked to a wide range of health benefits which include; digestive health, modulation of immune functions, antimicrobial properties etc [3,7]. Probiotics are present in the mucus membrane and on epithelial cells of the gut [8] and are known to release some antimicrobial compounds and compete with pathogens that lead to increase in the immune response of the host [9]. There are reports on the therapeutic effects of probiotics in diseases such as diarrhea, irritable bowel syndrome and Helicobacter pylori infection [6]. With the increasing evidences regarding safety and usefulness of probiotics, these bacteria are replacing the traditional prophylactic and treatment regimes.
The Lactobacillus genus is evolving and contains over 80 species. They are one of the safe and natural living probiotic bacteria as described by Florou-Paneri et al., [10] and are present in raw milk and dairy products such as cheeses, yoghurts, fermented milks as well as in natural sources. L. acidophilus has been specifically reported to act as a microbial barrier against several pathogens through competition for binding sites; enhancement of the host’s immune response, and production of antimicrobial substances including acids, bacteriocins and bacteriocins-like compounds [11].
As at the time of this study, there are limited reports on the evaluation of probiotic properties of microorganisms isolated from wine, fowl gut and rotten fruits, and their therapeutic effects. It is worthy to note that, majority of the known and reported probiotics are isolates from dairy products, and cannot be assimilated by lactose intolerant persons and thus, the need to evaluate isolates from other indigenous and natural sources. Therefore, this study was designed to isolate and characterize probiotics from palm wine, rotten banana fruit, and fowl gut and to evaluate the antidiarrhoeal and immunomodulatory effect of the isolates.
Methods
Sample collection
The probiotic bacteria were isolated from fresh palm wine, fowl gut and rotten banana fruits. Early morning fresh palm wine was purchased at Igboukwu, Anambra State. Banana fruits were bought from the market and allowed to rot. Three old layer fowls were sacrificed and samples aseptically collected from their guts. All the samples were transferred immediately to the laboratory for microbiological analysis and stored aseptically in low refrigerator temperature (-4°C) to protect normal flora and avoid contamination and deterioration.
Isolation and identification of bacteria
For the isolation of Lactobacillus species, 0.1ml aliquot of the serially diluted samples was aseptically spread on the surfaces of MRS (de Man, Rogosa, and Sharp) agar (Oxoid, England) plates. The inoculated plates were incubated under anaerobic condition using an anaerobic jar at 37°C for 48 hours. Then, 10 to 20 distinct colonies were randomly picked from countable MRS plates for further purification. The isolated colonies of lactic acid bacteria (LAB) were transferred into a 5 ml MRS broth (Oxoid) and purified by repeated streaking on MRS agar. Pure cultures of LAB isolates were then streaked on to slants of MRS agar and stored at +4°C for further characterization. The pure cultures of the organisms obtained were identified using Gram staining, colonial description, and biochemical reactions [12].
In vitro characterization of probiotic properties of isolates
Culture on MRS agar
Growths at 30, 37 and 45°C were observed in MRS broth under anaerobic conditions. The development was assessed by reading the optical densities at 570 nm every 2 h. For pH assay, the cultures were developed on MRS medium at various pH ranges: 3, 5.5, 6.5, 7, 7.5 and 8. After incubation on MRS agar for 24 h, isolates formed white, raised and creamy colonies.
Tolerance to low pH
0.1 ml aliquots of active overnight cultures of isolated LAB were each adjusted to a pH 3.0 and 2.0 using HCl and incubated at 37°C for 3 hours. Samples were then taken every hour for the next 3 hours, and the viable numbers of bacteria enumerated by pour plate counts of all samples using 10- fold serial dilutions prepared in 0.1% peptone water [13]. Simultaneously, bacterial growth was monitored by measuring absorbance with a spectrophotometer at 600 nm [14]. All the experiments were performed in triplicate.
Experimental animals for In Vivo studies
Healthy Swiss albino mice of either sex weighting 15-25 g and aged 5-6 weeks were used for the experiment. The animals were kept in pathogen free cages at room temperature and on a 12 h light/dark cycle. All the animals were regularly fed standard diet and water. All the mice were acclimated for one week prior to the experiment. During the acclimatization period and throughout the experimental periods, the animals were handled in accordance with established guidelines by Veterinary Surgeon Act Cap V3 LFN 2004, Federal Republic of Nigeria, and Animal Diseases (Control) Act. Cap A17 LFN, 2004, Federal Republic of Nigeria and National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals.
Anti-diarrhea evaluation of the isolated LAB
The anti-diarrhea activity of the isolated LAB was carried out as described by Igboeli et al., [15]. A total of 25 mice placed in five different groups consisting of 5 mice per group were used. The animals were five months old and weighed between 21-28 grams. All the animals were starved, having access to only water for 24 hours prior to the experimental day. Group 1 animals (serving as the negative control) received 10 ml/ kg of normal saline; Group 2 (the positive control) received 4 mg/kg of loperamide; Group 3 received 0.2 ml of isolate from banana; Group 4 received 0.2 ml of isolate from fowl gut; Group 5 received 0.2 ml of isolate from palm wine. One hour after the treatment, diarrhea was induced in all the animals by single administration of 1ml of castor oil orally (P.O). The animals were observed from the time of onset of diarrhea. Number of wet and solid feces was taken note of from each animal at 1hr, 2hr, 3hr, and 4hr post induction. The results were expressed as mean ± standard error of mean (SEM) and percentage diarrhea inhibition calculated using the relation:

Where a = mean total of number of wet stool for control.
And b = mean total of number of wet stool for treated group.
Gastrointestinal (GIT) motility study
GIT motility study was done using animal model. A total of 25 mice were used. The animals were starved for 24 hours prior to the experiment, having access to water alone. They were grouped into 5 groups, consisting of 5 mice per group. Group 1 animals (serving as the negative control), received 10 mg/ kg distilled water (PO); Group 2 received 10 mg/kg Atropine (IP) (Reference drug); Group 3 received 0.2 ml Banana isolate (PO); Group 4 received 0.2 ml fowl gut isolate (PO); Group 5 received 0.2 ml palm wine isolate (PO). Immediately, the animals were given 0.5 ml of 5% charcoal meal in mucilage of tragacanth. Fifteen minutes post treatment, the animals were sacrificed, dissected and their intestine cut loosed from the mesentery and layed outside. The distance the charcoal plug travelled from the pyloric region to the caeseum was measured and expressed as percentage of the entire length of the intestine.
Evaluation of the immunomodulatory effect of the probiotic bacteria
The immunomodulatory effect of the isolates was carried out using the method employed by Wahabet al. [16] A total of 30 adult albino mice were used. They were placed into 6 groups of 5 mice per group. The immune system of all the animals in each group was suppressed with single intra peritoneal administration of 75 mg/kg cyclophosphamide except the normal control group. Blood sample was collected from each animal and was analyzed for total white blood cell and differential count and was termed pre-treatment. Group 1 (serving as the negative control) received 10 mg/kg distilled water (P.O); Group 2 (positive control) received 100 mg/kg levamisol (P.O); Group 3 received 0.2 ml Banana isolate; Group 4 received 0.2 ml fowl gut isolate; Group 5 received 0.2 ml palm wine isolate; Group 6 normal control without suppression.
Statistical analysis
All the measurements were performed in triplicate, and the results expressed as mean standard deviation (SD). Data were analyzed by the one-way ANOVA using SPSS version 20 and graphs plotted using Microsoft Excel 2016. Statistical significance was considered at p ≤ 0.05.
Results
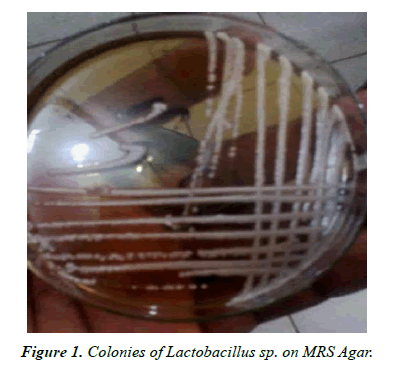
Figure 1 shows the morphological appearance of isolated LAB in this study. The plate showed isolates with white, creamy and raised colonies which conform to the colonial morphological description of most lactobacillus. Bacterial characteristics presented the (Table 1), show that, all the isolated bacteria were Gram positive, catalase negative, rod shaped, and oxidase negative, etc. All the bacteria were tolerant to harsh condition at low pH. All the results obtained showed that the bacteria were typical Lactobacillus spp.
| Isolates | Sources | ||
|---|---|---|---|
| Banana | Fowl Gut | Palm Wine | |
| Colonial Morphology | White, creamy and raised | White, small, creamy | White, creamy and raised |
| Microscopy | Rod shaped | Short rods | clusters of rods |
| Gram Staining | + | + | + |
| Catalase | - | - | - |
| Oxidase | - | - | - |
| Indole | - | - | - |
| Citrate | - | - | - |
| Glucose | + | + | + |
| Sucrose | + | + | + |
| Lactose | + | + | + |
Table 1. Biochemical and Physiological characteristics of isolated Bacteria.
Anti-diarrhea evaluation
Result obtained in the castor oil induced diarrheal activity revealed that loperamide, banana isolate, and fowl gut isolate displayed a time dependent activity, totally inhibited the discharge of wet stool in the first hour (Table 2). Also, all the tested probiotic isolates significantly inhibited the number of wet stool produced by the animals. Among the isolates, banana pill isolates showed higher activity, with no significant difference from inhibition percentage of the standard drug loperamide (p > 0.05).
| Groups | 1 hour | 2 hours | 3 hours | 4 hours | Total | % Inhibition |
|---|---|---|---|---|---|---|
| 10 mg/kg Distilled water | 1.67 ± 0.14 | 3.00 ± 0.58 | 1.67 ± 0.33 | 1.33 ± 0.33 | 7.67 ±2.44 | 0 |
| 4 mg/kg loperamide | 0.0 0.00 | 1 ± 0.06 | 1 ± 1.00 | 0.67 ± 0.33 | 2.67 ± 2.33 | 69.19 |
| 0.2 ml Banana isolate | 0.0 ± 0.00 | 2.67 ± 0.33 | 1 ± 0.00 | 0.67 ± 0.33 | 4.34 ± 0.66 | 43.41 |
| 0.2 ml Fowl gut isolate | 0.0 ± 0.00 | 1.67 ± 1.20 | 2 ± 1.15 | 1 ± 0.57 | 4.67 ± 2.92 | 39.11 |
| 0.2 ml Palm wine isolate | 1.33 ± 1.33 | 1.67 ± 0.88 | 2 ± 0.58 | 0.33 ± 0.33 | 5.33 ± 3.12 | 30.51 |
Table 2. Mean number of wet stool.
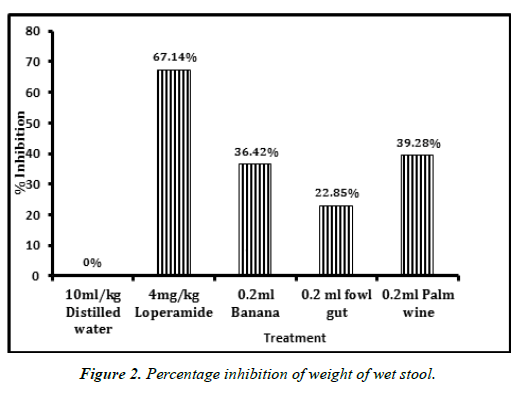
Isolated probiotics caused significant time dependent reduction in the mean weight of wet stool produced (Figure 2). Palm wine isolates was observed to show the highest inhibition (39.28%), only bettered by the positive control Loperamide (67.14%). No inhibition was observed in the case of distilled water.
The mean delay and percentage delay of diarrhea onset is presented in (Table 3). All the test isolates delayed the onset of diarrhea, with isolate from banana showing the highest activity (48.36%). The banana isolate showed a statistically significant (p < 0.05) effect when compared to the negative control. However, there was no significant difference observed when compared with the positive control Loperamide. Among the probiotic isolates tested, isolates from fowl gut displayed the shortest delay time of 80 ± 0.41 min, but was still observed to be longer than that of the negative control (63 ± 0.22).
| Group | Mean delay (in minutes) | % delay |
|---|---|---|
| 10mg/kg Distilled water | 063 ± 0.22 | 0 |
| 4mg/kg loperamide | 157 ± 0.94* | 59.87 |
| 0.2 ml Banana isolate | 122 ± 0.08* | 48.36 |
| 0.2 ml Fowl gut isolate | 080 ± 0.41 | 21.25 |
| 0.2 ml Palm wine | 095 ± 0.56 | 33.68 |
Table 3. Mean time delay and percentage time delay.
The results represent three independent experiments. Results are expressed as mean ±SD. significantly different from negative control *(P <0.05).
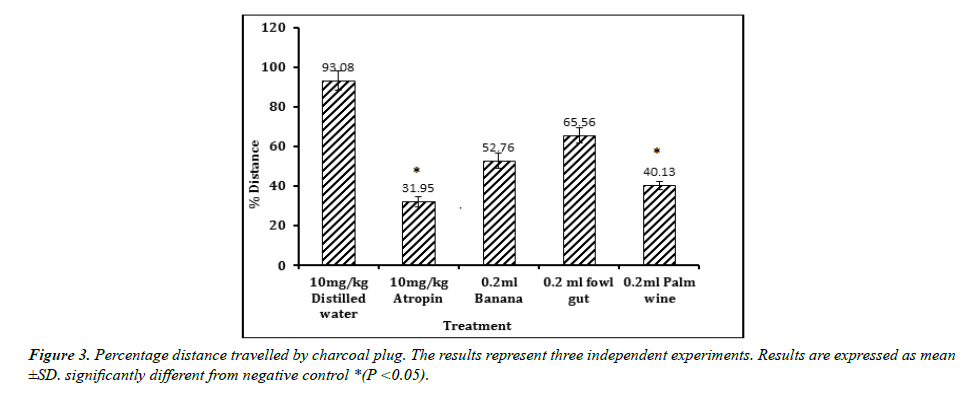
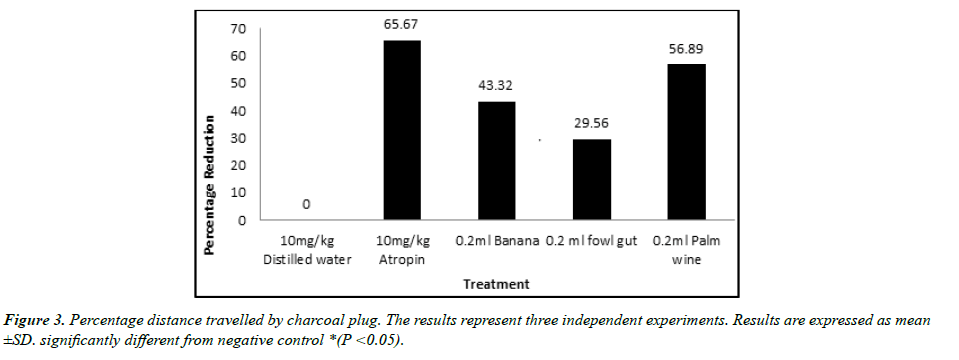
The result of GIT motility study is shown in figures. Result obtained reveal that a single dose administration of the test isolates was able to cause a significant reduction in the distance traveled by charcoal meal (Figure 3). The shortest distance was displayed by the positive control Atropine (31.95), followed by isolate from palm wine (40.13). Both showed a significant (p < 0.05) effect when compared with the negative control (93.08).A similar result was observed in the percentage reduction in GIT motility (Figure 4). Atropine (65.67%) and isolate from palm wine (56.89%) gave a higher reduction than other isolates. No activity was recorded in the group treated with distilled water.
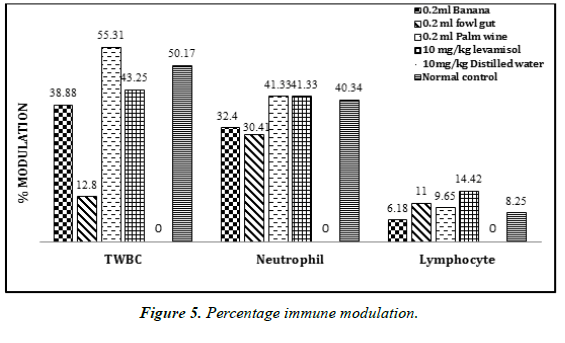
The mean white blood cell (WBC) and differential count is presented in (Table 4). There was an increase in the level of mean WBC count and differential count after the administration of probiotics from the different sources. Probiotic bacteria isolated from palm wine (3.2 ± 0.23) showed a higher WBC count than the standard drug levamisole (2.52 ± 0.31). However, all the isolated probiotic organisms showed a reasonable increase in the level of total WBC, differential count, and percentage immune modulation (Figure 5), when compared to the negative control.
| Groups | TWBC x 10g/l | Neutrophils | Lymphocytes | Basophils | Eosiniphils | Monocytes |
|---|---|---|---|---|---|---|
| Banana isolate | 2.34 ± 0.32 | 35 ± 1.53 | 63 ± 2.08 | 0.33 ± 0.33 | 1.33 ± 0.67 | 3 ± 0.00 |
| Fowl gut isolate | 1.64 ± 0.24 | 34 ± 1.45 | 66.67 ± 0.88 | 0 ± 0.00 | 1 ± 0.58 | 2.3 ± 1.20 |
| Palm wine isolate | 3.2 ± 0.23 | 40.3 ± 0.88 | 65.67 ± 2.60 | 0. 67 ± 0.33 | 1 ± 0.58 | 3.67 ± 0.33 |
| Levamisol | 2.52 ± 0.31 | 40.33 ± 1.86 | 69.33 ± 0.88 | 0.67 ± 0.33 | 0.67 ± 0.67 | 3.33 ± 0.33 |
| Distiled water | 1.43 ± 0.18 | 23.66 ± 1.45 | 59.33 ± 1.20 | 0.0 ± 0.00 | 0.33 ± 0.33 | 3 ± 0.00 |
| Normal control | 2.87 ± 0.08 | 39.66 ± 1.20 | 64.67 ± 1.76 | 0.67 ± 0.33 | 1 ± 0.58 | 3.33 ± 0.33 |
Table 4. Mean WBC and differential count.
Discussion
In this study, the antidiarrheal and immunomodulatory evaluations of probiotics isolated from palm wine, fowl gut and rotten banana fruit was investigated. Probiotics were isolated using de Man Rogosa and Sharpe (MRS) media, whose components are known to serve as special growth factors for lactic acid bacteria growth and survival [17]. All the isolates were considered probiotic bacteria based on their colony morphology and biochemical characteristics. Isolated probiotic organisms were identified as Lactobacillus species based on their cultural and biochemical characteristics. Similarly, Hoque et al. [18] by observing their morphological and different biochemical characteristics reported the identification of Lactobacillus species from yogurt samples. All the bacteria were tolerant to harsh condition at low pH after 3 hours. Resistance to low pH is one of the key characteristics of probiotics [17].
Diarrheal disease is a major health concern, as it is regarded as a leading cause of mortality and morbidity, especially among children in the developing countries [19]. Diarrhea is usually a symptom of an infection in the intestinal tract, which can be caused by several infectious agents or as a result of excessive use of broad-spectrum antibiotics as in antibiotic associated diarrhea. Evidence obtained from different animal models used in this study substantiates the use of probiotics in the management of diarrhea and its associated pain. A strong antidiarrheal effect of the probiotics in the castor oilinduced diarrhea model was observed. This is in correlation with reports by other researchers using the probiotic yeast Sacchamomycesboulardii. In the study, a significant antidiarrhoeal effects were observed at the dose of 12 × 1010 CFU/kg, which is four times more than the dose of bacteria administered in this study [20,21]
Interestingly, probiotics isolated in this study significantly inhibited diarrhea without completely blocking defecation, whereas loperamide, the reference drug, both stopped diarrhea (100%) and defecation. The blockage of defecation could, in part, explain the pain observed in mice treated with loperamide. Indeed, the global behavioral score observed in this study with loperamide suggests pain sensation in mice treated with the reference drug. In contrast, the results we observed with the probiotic mixture are in favours of an anti-nociceptive effect. The analgesic effect of the same LAB probiotic has previously been reported in a clinical study in the United States [22].
Barai et al, [17] reported that Probiotics exhibiting antidiarrheal activity may have a potential to retard the onset of diarrhea significantly. All the probiotics test isolates delayed the onset of diarrhea, with isolate from banana showing the highest activity (48.36%). The banana isolate showed a statistically significant (p < 0.05) effect when compared to the negative control. A decrease in the consistency and an increase in frequency in bowel movements to greater than 3 stools per day generally describes diarrhea, according to the WHO criteria. Giduduet al., (2010) defined diarrhea, when the water percentages exceed 90% whereas the water percent of stools is normally 60~90%.
Castor oil is known to induce diarrhea through its active metabolite, ricinoleic acid [23]. In the intestine, ricinoleic acid induces inflammation and irritation of the mucosa and the release of mediators like prostaglandins that prevent fluid and electrolyte absorption and increase the intestinal peristaltic movements. These events culminate to diarrhea [24-26]. By using the castor oil-induced enter pooling model and the charcoal transit method; we showed that the probiotic isolates decreased intestinal motility. The results thus suggest that the probiotic bacterial strains have strong anti-diarrheic through anti secretory properties.
We previously reported that probiotics, including the Lactobacilli genus modulates the innate immune response, macrophages and inflammatory responses [3].The intestinal micro biota acts as primary agent in the developing the postnatal immune system. The interaction of probiotics and enterocytes is crucial in immunomodulation. Probiotics act on a various cell in the intestine to modulate the immune system. The strain, setting and immunological parameters measured and the type of cells being acted upon all influence the degree of immuno-modulation [27].
Probiotic bacteria isolated from palm wine (3.2 ± 0.23) showed a higher WBC count than the standard drug levamisole (2.52 ± 0.31) used in this study. However, all the isolated probiotic organisms showed a reasonable increase in the level of total WBC, differential count (Table 4), and percentage immune modulation have been found in many types of fruit juices from both solid and citrus fruits whereas Leuconostoc mesenteroides is most commonly found in tomatoes. It was observed that probiotics isolated from these natural sources was able to cause a significant increase in the level of total WBC and differential count in laboratory mice after cyclophosphamide induced immunosuppression [28]. This agrees with the findings in this current study. This is also in line with a previous work done by Fang et al., who reported that the immune system can be modulated through the intake of probiotics.
There are emerging evidences in favours of the claims on the beneficial effects exerted by probiotics, which includes enhancement of the immune response, prevention and treatment of gastrointestinal disorders such as antibiotic associated-diarrhoea, inflammatory bowel diseases. Probiotics shows promising approach to prevent chronic Inflammatory Bowel Disease (IBD) because of their immunomodulatory and bowel flora correcting properties, but the beneficial effect of probiotics on IBD have yet to reach the high expectations predicted by mechanistic and animal studies, particularly for Crohn’s disease.
Conclusion
Probiotic bacteria present in natural resources such as palm wine, fowl gut and rotten banana fruit possess both antidiarrhoeal and immunomodulatory properties. Due to the promising activities displayed by Lactobacillus species, these isolates can serve as potential useful organisms for the formulation probiotic products. However, further studies are still needed to determine the precise action modes of probiotics on both mucosal and systemic immunity.
Conflict of Interest
None
Author’s Contribution
Design of the study: AA Attama & MC Ugwu
Acquisition, analysis, or interpretation of data for the work: CJ Ezeibe & DC NwobodoSupervision: Malachy C. Ugwu and Anthony A Attama.
Drafting and or revising for intellectual content: NT Ujam, CB Ugwu, DC Nwobodo & MC Ugwu.
References
- Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. The Lancet. 2010;376(9741):606-14.
- Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. The lancet. 2010;375(9730):1969-87.
- Nwobodo DC, Ugwu MC. Immunomodulatory Potentials of Probiotics: A Review. Asian J. Immunol. 2020;3:1-5.
- Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35-7.
- Macfarlane S, Furrie E, Cummings JH, et al. Chemotaxonomic analysis of bacterial populations colonizing the rectal mucosa in patients with ulcerative colitis. Clinical Infec Dis. 2004;38(12):1690-9.
- Terpou A, Papadaki A, Lappa IK, et al. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nut. 2019;11(7):1591.
- Filidou E, Kolios G. Probiotics in Intestinal Mucosal Healing: A New Therapy or an Old Friend?. Pharma. 2021;14(11):1181.
- Soccol CR, de Souza Vandenberghe LP, De Dea Lindner J, et al. The potential of probiotics: a review.
- Flourou-Paneri P, Christaki E, Bonos E. Lactic Acid Bacteria as Source of Functional Ingredients: Lactic Acid Bacteria–R & D for Food. London: IntechOpen. 2013.
- Shokri D, Khorasgani MR, Mohkam M, et al. The inhibition effect of lactobacilli against growth and biofilm formation of Pseudomonas aeruginosa. Probiotics and Antimicrobial Proteins. 2018;10(1):34-42.
- Nworah I, Umeaku C. Antimicrobial activities of Vernonia amygdalina, Ocimum gratissimum and Garcinia kola. Int J Res Med Basic Sci. 2016;6(2):1-2.
- Degnan FH. The US Food and Drug Administration and probiotics: regulatory categorization. Clinical inf dis. 2008;46:S133-6.
- Rohit S, Khaleel A, Santani DD. Reporting and monitoring of adverse drug reactions with cardiac drugs. IRJP. 2011;2:116-9.
- Igboeli N, Onyeto CA, Okorie AN, et al. Antidiarrheal activity of methanol leaf extract of Lophira Lanceolata Tiegh (Ochnaeceae). Merit Res J Environ Sci Toxicol. 2015;3(4):059-64.
- Wahab S, Hussain A, Farooqui AH, et al. In vivo antioxidant and immunomodulatory activity of Bombax ceiba bark-Focusing on its invigorating effects. Am J Adv Drug Delivery. 2014;2:1-3.
- Barai P, Hossain KM, Rahman SM, et al. Antidiarrheal efficacy of probiotic bacteria in castor oil induced diarrheal mice. Prev Nut Food Sci. 2018;23(4):294.
- Hoque MZ, Akter F, Hossain KM, et al. Isolation, identification and analysis of probiotic properties of Lactobacillus spp. from selective regional yoghurts. World J Dairy Food Sci. 2010;5(1):39-46.
- Ugwu MC, Edeani GI, Ejikeugwu CP, et al. Antibiotic susceptibility profile of Escherichia coli and Salmonella causing childhood diarrhoea in Awka Municipality, South-Eastern Nigeria. Clin Microbiol 2017;6(277):2.
- Drouault-Holowacz S, Bieuvelet S, Burckel A, et al. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastro enterologie clinique et biologique. 2008;32(2):147-52.
- Holowacz S, Blondeau C, Guinobert I, et al. Antidiarrheal and antinociceptive effects of a probiotic mixture in rats. J Prob Health. 2016;4(3).
- Salako OA, Akindele AJ, Shitta OM, et al. Antidiarrhoeal activity of aqueous leaf extract of Caladium bicolor (Araceae) and its possible mechanisms of action. J Ethno pharmacol. 2015;176:225-31.
- Sharma DK, Gupta VK, Kumar S, et al. Evaluation of antidiarrheal activity of ethanolic extract of Holarrhena antidysenterica seeds in rats. Veterinary World. 2015;8(12):1392.
- Yakubu MT, Nurudeen QO, Salimon SS, et al. Antidiarrhoeal activity of Musa paradisiaca Sap in Wistar rats. Evidence-Based Complementary and Alternative Med. 2015.
- Azad M, Kalam A, Sarker M, et al. Immunomodulatory effects of probiotics on cytokine profiles. Bio Med Res Int. 2018.
- Naeem M, Ilyas M, Haider S, et al. Isolation characterization and identification of lactic acid bacteria from fruit juices and their efficacy against antibiotics. Pak J Bot. 2012;44(323):8.
- Fang H, Elina T, Heikki A, et al. Modulation of humoral immune response through probiotic intake. FEMS Immunol Med Microbiol. 2000;29(1):47-52.
- Shanahan F, Collins SM. Pharmabiotic manipulation of the microbiota in gastrointestinal disorders, from rationale to reality. Gastroenterol Clinics. 2010;39(3):721-6.
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterol. 2008;134(2):577-94.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref