Research Article - Biomedical Research (2016) Volume 27, Issue 4
Anticancer evaluation of novel 1,3,4-trisubstituted pyrazole candidates bearing different nitrogenous heterocyclic Moieties
Magda M. F. Ismail1, Nagy M. Khalifa2,3*, Hoda H. Fahmy3, Hend M. EL-Sahrawy1 and Eman S. Nossier11Department of Pharmaceutical Chemistry, Faculty of Pharmacy (Girls), Al-Azhar University, Cairo, Egypt
2Drug Exploration & Development Chair (DEDC), College of pharmacy, King Saud University, Riyadh 11451, Saudi Arabia
3Department of Therapeutically Chemistry, Pharmaceutical and Drug Industries Division, National Research Centre, Giza, Egypt
- *Corresponding Author:
- Nagy Khalifa
Drug Exploration & Development Chair, King Saud University, Saudi Arabia
Accepted on March 28, 2016
Abstract
With the goal of developing novel and potent anticancer therapies, a new set of 1,3,4-trisubstituted pyrazole derivatives linked to different heteroaryl systems at C-4 position were prepared and estimated for their anticancer effects at NCI, USA employing a two stage procedure using 60 various human tumor cell lines. Anticancer evaluation disclosed that, compound 4c displayed super fat potency towards most human tumor cell lines with GI50 (0.52-5.15 μM), particularly against colon SW-620 cell line with GI50 (0.52 μM). At the same time, 4c was also selective against A498 and RXF 393 cell lines of renal cancer with GI50 0.58 and 0.86 μM respectively.
Keywords
Anticancer evaluation, 1,3,4-trisubstituted pyrazoles, Synthesis.
Introduction
Cancer is a main health issue expressible as a leading reason of doom [1] and the current therapies for cancers handling are unsatisfactory due to the comparatively significant side effects [2]. So, the development of new anticancer therapies with higher specificity and depress poisoning is of great interest [3]. In deal for best antitumor therapy, great sets of pyrazoles were obtained and screened from long years. The pyrazole scaffold performs a substantial class in many pharmaceutical active compounds and estimated in Phase I considerations as an antitumor factor in human being. Even in daily reduced dosages it demonstrated likewise venomous for human employ because of evolution of signals of hepato-toxicity [4]. In trying to defeat this passive signs, an integration of pyrazole rings with several heteroaryl systems was notified to display considerable anticancer properties [5-10]. Furthermore, literature scanning disclosed that several pyrazole compounds have been performed as antiproliferative, antitumor and antileukemic. Also, these products are eligible to extend marked anti-cancer properties through suppression of various kinds of enzymes and proteins which afford crucial functions in cell segmentation [11]. In addition, substituted pyrazoles are also examined for their antiproliferative properties in vitro and antitumor action in vivo, with hopeful command compounds [12-14]. In the same direction, and as a part of our outstanding research agenda in developing new cytotoxic products [15-17], we prepared novel polysubstituted pyrazole compounds bearing various nitrogenous heterocyclic rings and their anticancer activities were estimated.
Experimental
Melting degrees were established using open capillary tubes with Griffin device and are uncorrected. Elemental microanalyses were set in the agreeable ends of the elaborated rates. IR spectra (ν, cm–1) using KBr discs were listed by Schimadzu 435 IR Spectrophotometer. NMR spectra (DMSOd6) δ, ppm) were measured using Varian Gemini 500 MHz and Brucker 500 MHz Spectrophotometer with (TMS) as interior criterion device. Mass Spectra (EI, 70 eV) were recorded on Hewlett Packard 5988 device. TLC-analysis was carried out on silica gel aluminum slabs, 60 F254 for reactions progress.
2-((1-(m-Chlorophenyl)-3-(p-methoxyphenyl)-1Hpyrazol- 4-yl)methylene)malononitrile (2)
A mix of pyrazole-4-carboxaldehyde 1 (0.01 mol), malononitrile (0.01 mol) and drops of piperidine in ethanol (40 mL) was heated at 70°C for 1 h and then poured onto water. The resulting solid precipitate was filtered and purified from EtOH to give compound 2 in 86% yield, mp 140-143°C; IR, ν: 3133 (CH-Ar), 2224 (2C≡N); 1H-NMR: δ 3.88 (s, 3H, OCH3), 7.08-8.14 (m, 9H, ArH + CH), 9.23 (s, 1H, CH); MS: m/z (%): 362 (3.45), 360 (9.29), 80 (100); Anal. Calcd for C20H13ClN4O: C, 66.58; H, 3.63; N, 15.53; Found: C, 66.61; H, 3.59; N, 15.55.
1,6-Diamino-4-[1-(m-chlorophenyl)-3-(pmethoxyphenyl)- 1H-pyrazol-4-yl]-2-oxopyridine-3,5- dicarbonitrile (3)
A mix of freshly prepared 2-cyanohydrazide (0.02 mol) and compound 2 (0.01 mol) in ethyl alcohol containing piperidine (25 mL) was refluxed for 1 h. The precipitate formed was collected, filtered, washed with ethanol and purified from MeOH to give compound 3 in 75% yield, mp 263-265 oC; IR, ν: 3133 (CH-Ar), 3391, 3276 (2NH2), 2210 (2C≡N), 1750 (CO); 1H-NMR: δ 3.80 (s, 3H, OCH3), 5.68 (s, 2H, NH2 commutable), 7.01-8.08 (m, 8H, ArH), 8.49 (s, 2H, NH2 commutable), 9.06 (s, 1H, CH); 13C-NMR: δ 55.08, 75.61, 114.17, 115.33, 116.26, 116.79, 118.00, 124.12, 126.67, 128.28, 129.84, 131.55, 134.28, 139.99, 149.91, 151.74, 156.62, 159.25, 159.62, 168.00; MS: m/z (%): 459 (37.99), 457 (100); Anal. Calcd for C23H16ClN7O2 (457.5): C, 60.33; H, 3.52; N, 21.41; Found: C, 60.37; H, 3.48; N, 21.36.
7-(1-(m-Chlorophenyl)-3-(p-methoxyphenyl)-1Hpyrazol- 4-yl)-2-substituted-5-oxo-triazolo- [1,5- a]pyridine-6,8-dicarbonitriles (4a-e)
An equimolar mix of 3 (0.01 mol) and various aldehydic compounds namely, 5-methylfuran-2-carboxaldehyde, benzaldehyde, 4-tolylaldehyde, 4-dimethylamino benzaldehyde or 4-methoxybenzaldehyde (0.01 mol) in absolute ethyl alcohol containing piperidine (50 mL) were refluxed for 6-8 h. the solid formed was filtered off, dried and purified from AcOH to give compounds 4a-e.
7-(1-(m-Chlorophenyl)-3-(p-methoxyphenyl)-1Hpyrazol- 4-yl)-2-(5-methyl furan-2-yl)-5-oxotriazolo[ 1,5-a]pyridine-6,8-dicarbonitrile (4a)
Yield 69%; mp 205-207°C; IR, ν: 3424 , 3305 (2 NH), 2215 (2C≡N), 1667 (CO); 1H-NMR: δ 2.49 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 6.50 (d, 1H, CH), 6.95-8.10 (m, 10H, ArH), 8.40 (br, 2H, 2NH, commutable), 9.10 (s, 1H, CH); 13C-NMR: δ 13.74, 55.09, 78.22, 85.43, 109.94, 114.20, 116.78, 117.20, 117.99, 120.95, 123.86, 124.22, 126.65, 127.62, 128.29, 129.85, 131.52, 134.21, 141.45, 150.36, 155.48, 159.34, 161.67, 173.00; MS: m/z (%): 551 (1.98), 549 (3.77), 53 (100); Anal. Calcd for C29H20ClN7O3 (549.5): C, 63.33; H, 3.67; N, 17.83; Found: C, 63.60; H, 3.28; N, 17.83.
7-(1-(m-Chlorophenyl)-3-(p-methoxyphenyl)-1Hpyrazol- 4-yl)-5-oxo-2-phenyltriazolo[1,5- a]pyridine-6,8-dicarbonitrile (4b)
Yield 65%; mp 246-248°C; IR,ν: 3418, 3366 (2 NH), 2216 (2C≡N), 1674 (CO); 1H-NMR: δ 3.81 (s, 3H, OCH3), 7.02 (d, 1H, CH), 7.49-8.12 (m,13H, ArH), 8.52 (br, 2H, 2NH, commutable), 9.09 (s, 1H, CH); 13C-NMR: δ 55.08, 75.89, 88.50, 114.28, 115.13, 116.13, 116.87, 118.07, 124.04, 126.72, 128.40, 128.89, 129.23, 129.86, 131.57, 131.78, 133.35, 134.28, 139.99, 149.98, 151.99, 154.03, 156.30, 159.63, 172.83; MS: m/z (%): 547 (1.94), 545 (4.89), 103 (100); Anal. Calcd for C30H20ClN7O2: C, 66.00; H, 3.69; N, 17.96; Found: C, 66.27; H, 3.40; N, 18.09.
7-(1-(m-Chlorophenyl)-3-(p-methoxyphenyl)-1Hpyrazol- 4-yl)-5-oxo-2-p-tolyltriazolo[1,5-a]- pyridine-6,8-dicarbonitrile (4c)
Yield 63%; mp 242-244 oC; IR,ν: 3417, 3361 (2 NH), 2215 (2C≡N), 1674 (CO) cm-1; 1H-NMR: δ 2.43 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 7.03 (d, 1H, CH), 7.39-8.11 (m, 12H, ArH), 8.49 (br, 2H, 2NH, commutable), 9.09 (s, 1H, CH) ppm; 13CNMR: δ 21.34, 55.15, 75.86, 88.52, 114.28, 115.04, 116.15, 116.79, 118.06, 124.05, 126.8, 127.02, 128.39, 129.27, 129.90, 131.57, 134.28, 139.99, 143.81, 149.97, 151.90, 153.08, 154.03, 156.37, 159.62, 172.62 ppm; MS: m/z (%): 561 (0.63), 559 (9.89), 444 (22.97), 442 (100); Anal. Calcd for C31H22ClN7O2: C, 66.49; H, 3.96; N, 17.51; Found: C, 66.68; H, 3.66; N, 17.64.
7-(1-(m-Chlorophenyl)-3-(p-methoxyphenyl)-1Hpyrazol- 4-yl)-2-(4-(dimethylamino)phenyl)- 5-oxo- [1,2,4]triazolo[1,5-a]pyridine-6,8-dicarbonitrile (4d)
Yield 66%; mp 272-274°C; IR, ν: 3356, 3273 (2 NH), 2216 (2C≡N), 1669 (CO); 1H- NMR: δ 3.06 (s, 6H, 2CH3), 3.81 (s, 3H, OCH3), 6.83 (d, 1H, CH), 7.03- 8.11 (m, 12H, ArH), 8.37 (br, 2H, 2NH, commutable), 9.08 (s, 1H, CH); 13C-NMR: δ 39.54, 55.14, 75.73, 88.56, 111.22, 114.28, 115.13, 116.27, 116.83, 118.04, 118.47, 124.07, 126.68, 128.35, 129.85, 131.56, 131.68, 134.27, 140.00, 149.94, 152.00, 153.57, 154.04, 156.72, 159.61, 171.85; Anal. Calcd for C32H25ClN8O2: C, 65.25; H, 4.28; N, 19.02; Found: C, 65.51; H, 4.01; N, 19.12.
7-(1-(m-Chlorophenyl)-3-(p-methoxyphenyl)-1Hpyrazol- 4-yl)-2-(4-methoxyphenyl)-5-oxotriazolo[ 1,5-a]pyridine-6,8-dicarbonitrile (4e)
Yield 62%; mp 244-246 oC; IR, ν: 3429, 3304 (2 NH), 2216 (2C≡N), 1673 (CO); 1H-NMR: δ 3.81, 3.88 (2s, 6H, 2 OCH3), 7.03 (d, 1H, CH), 7.14-8.11 (m, 12H, ArH), 8.47 (br, 2H, 2NH, commutable), 9.09 (s, 1H, CH); 13C-NMR: δ 55.15, 55.66, 75.83, 88.50, 114.29, 114.38, 115.07, 116.18, 116.85, 118.06, 124.05, 124.32, 126.72, 128.38, 129.90, 131.58, 131.98, 134.28, 139.99, 149.97, 151.78, 154.03, 156.45, 159.62, 163.41, 172.02; MS: m/z (%): 575 (1.34), 133 (100); Anal. Calcd for C31H22ClN7O3: C, 64.64; H, 3.85; N, 17.02; Found: C, 64.82; H, 3.54; N, 17.14.
2-Amino-4-(1-(m-chlorophenyl)-3-(pmethoxyphenyl)- 1-pyrazol-4-yl)quinoline-3- carbonitrile (5) and 2-Amino-4-(1-(m-chlorophenyl)-3-(p-methoxyphenyl)-1H-pyrazol-4- yl)-5-oxo-5H-indeno[1,2-b]pyridine-3-carbonitrile (6)
A mixture of 2 (0.01 mol), cyclohexanone or 1,3-indanedione (0.01 mol) and CH3COONH4+ (0.01 mol) in absolute ethyl alcohol (40 mL) was heated to reflux for 3-5 h. the residue created was clarified and purified from EtOH to output the designate products 5 and 6, respectively.
2-Amino-4-(1-(m-chlorophenyl)-3-(pmethoxyphenyl)- 1-pyrazol-4-yl)quinoline-3- carbonitrile (5)
Yield 72%; mp 202-205°C; IR, ν: 3321 (NH2), 2217 (C≡N); 1H-NMR: δ 1.50-2.68 (m, 8H, 4CH2), 3.81 (s,3H, OCH3), 4.87 (s, 2H, NH2 D2O commutable), 6.65-8.78 (m, 8H, ArH), 8.88 (s, 1H, CH); MS: m/z (%): 457 (36.74), 455 (100); Anal. Calcd for C26H22ClN5O: C, 68.49; H, 4.86; N, 15.36; Found: C, 68.44; H, 4.89; N, 15.32.
2-Amino-4-(1-(m-chlorophenyl)-3-(pmethoxyphenyl)- 1-pyrazol-4-yl)-5-oxo-5H-indeno[1,2- b] pyridine-3-carbonitrile (6)
Yield 76%; mp 220-222°C; IR,ν: 3429, 3283 (NH2), 2216 (C≡N), 1605 (C=O); 1H-NMR: δ 3.84 (s, 3H, OCH3), 6.65-8.82 (m, 12H, ArH), 9.14 (s, 1H, CH), 9.96 (s, 2H, NH2 commutable); MS: m/z (%): 505 (2.04), 503 (2.95), 382(100); Anal. Calcd for C29H18ClN5O2: C, 69.12; H, 3.60; N, 13.90; Found: C, 69.08; H, (3.57; N, 13.86.
7-Amino-5-(1-(m-chlorophenyl)-3-(p-methoxy phenyl)-1-pyrazol-4-yl) -4-oxo-2-oxo(thioxo)-1Hpyrano[ 2,3-d]pyrimidine-6-carbonitriles (7a,b)
A mixture of 2 (0.01 mol) and barbituric acid or thiobarbituric acid (0.01 mol) in absolute ethyl alcohol with drops of piperidine (40 mL) was refluxed for 1-2 h. The residue was refined and purified from ethanol to get the designed products 7a,b.
7-Amino-5-(1-(m-chlorophenyl)-3-(pmethoxyphenyl)- 1-pyrazol-4-yl)-2,4-dioxo-1H-pyrano [2,3-d]pyrimidine-6-carbonitriles (7a)
Yield 65%; mp 190-193°C; IR,ν: 3468, 3348, 3227(NH2, 2NH), 2222 (C≡N), 1701, 1631 (2CO); 1H-NMR: δ 3.82 (s, 3H, OCH3), 4.15 (s, 1H, CH), 6.33 (s, 2H, NH2 commutable), 6.95-7.91 (m, 8H, ArH),9.74 (s, 1H, CH), 11.16, 11.30 (2s, 2H, 2NH commutable); MS: m/z (%): 490 (0.14), 488 (0.14), 299 (33.27), 297 (100); Anal. Calcd for C24H17ClN6O4: C, 58.96; H, 3.50; N, 17.19; Found: C, 59.02; H, 3.43; N, 17.22.
7-Amino-5-(1-(m-chlorophenyl)-3-(pmethoxyphenyl)- 1-pyrazol-4-yl)-4-oxo-2-thioxo-1Hpyrano[ 2,3-d]pyrimidine-6-carbonitriles (7b)
Yield 67%; mp 110-112°C; IR,ν: 3415, 3348, 3121 (NH2, 2NH), 2220 (C≡N), 1718 (C=O); 1H-NMR: δ 3.34 (s, 2H, NH2 commutable), 3.38 (s, 1H, NH, commutable), 3.76 (s, 3H, OCH3), 4.16 (s, 1H, CH), 6.97- 7.84 (m, 8H, ArH), 7.97 (s, 1H, NH D2O commutable), 8.57 (s, 1H, CH); Anal. Calcd for C24H17ClN6O3S: C, 57.09; H, 3.39; N, 16.64; Found: C, 57.13; H, 3.34; N, 16.62.
Biological assay:
The protocol for NCI-60 anticancer screening has been adopted according to reported standard procedure [18-20].
Results and Discussion
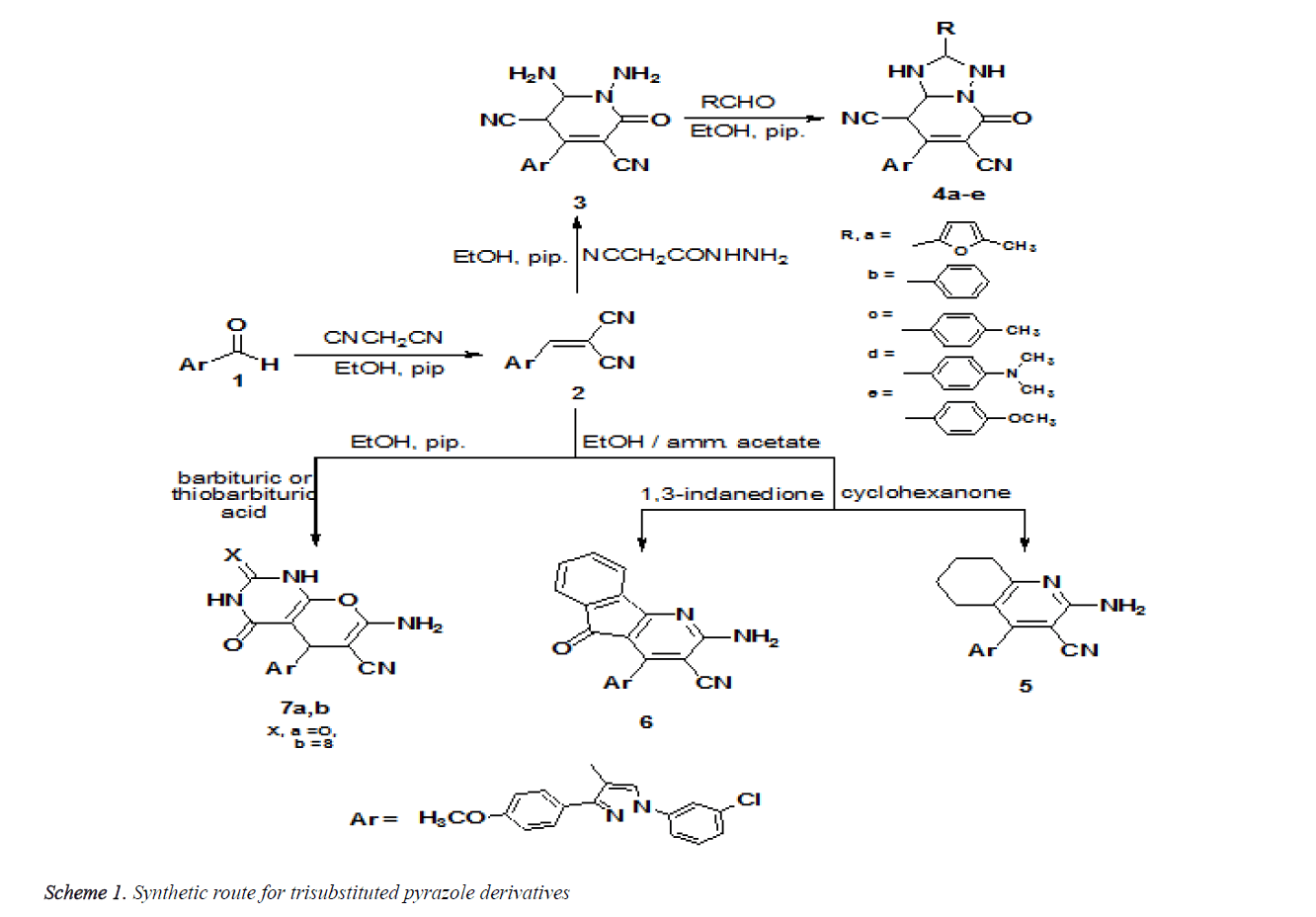
Synthesis of the required pyrazoles based on reaction of the starting material pyrazole-4-carboxaldehyde derivative 1 with malononitrile to afford the corresponding key intermediate pyrazole-4-methylene malononitrile 2. Reaction of 2 with freshly prepared 2-cyanohydrazide yielded 2-oxopyridine dicarbonitrile derivative 3. Treatment of 2 with diverse aldehydes in absolute ethyl alcohol with piperidine yielded triazolo [1,5-a] pyridine dicarbonitrile derivatives 4a-e. Reaction of the arylidene malononitrile 2 with cyclohexanone or 1,3-indandione in presence of ammonium acetate gave tetrahydroquinoline 5 and indeno[1,2-b]pyridine 6 derivatives, respectively. Pyrano [2,3-d] pyrimidines derivatives 7a,b, were prepared on reaction of compound 2 with barbituric or thiobarbituric (Scheme 1).
| Subpanel cell lines | ||||
|---|---|---|---|---|
| GI50 | ||||
| 2 | 4a | 4c | ||
| Leukemia | ||||
| CCRF-CEM | 1.43 | 2.45 | 1.23 | |
| HL-60(TB) | 2.39 | 4.88 | 8.34 | |
| K-562 | 2.01 | 4.09 | 1.43 | |
| MOLT-4 | 2.12 | 3.23 | 0.75 | |
| RPMI-8226 | 2.30 | 5.68 | 4.57 | |
| SR | 1.54 | 4.22 | 1.43 | |
| Non-Small Cell Lung Cancer | ||||
| A549/ATCC | 2.30 | 4.57 | 1.85 | |
| EKVX | 2.39 | 1.54 | 1.62 | |
| HOP-62 | 2.59 | 14.10 | 0.88 | |
| HOP-92 | 1.26 | 0.46 | 0.72 | |
| NCI-H226 | 2.74 | 7.35 | 2.39 | |
| NCI-H23 | 2.68 | 8.09 | 3.18 | |
| NCI-H460 | 2.05 | 6.38 | 1.85 | |
| NCI-H522 | 2.21 | 4.96 | ` | |
| NCI-H322M | ||||
| Colon Cancer | ||||
| COLO 205 | 4.04 | 5.47 | 2.32 | |
| HCC-2998 | 3.67 | 7.43 | 5.04 | |
| HCT-116 | 1.73 | 2.53 | 1.24 | |
| HCT-15 | 3.24 | 1.66 | 9.95 | |
| HT29 | 2.48 | 7.21 | 1.49 | |
| KM12 | 2.83 | 3.54 | 3.39 | |
| SW-620 | 1.90 | 9.21 | 0.52 | |
| CNS Cancer | ||||
| SF-268 | 2.50 | 5.23 | 1.62 | 2.50 |
| SF-295 | 2.31 | 3.46 | 1.20 | 2.31 |
| SF-539 | 2.30 | 5.66 | 1.28 | 2.30 |
| SNB-19 | 2.63 | 8.63 | 2.17 | 2.63 |
| SNB-75 | 1.81 | 1.56 | 0.83 | 1.81 |
| U251 | 1.59 | 4.71 | 0.80 | 1.59 |
| Melanoma | ||||
| LOX IMVI | 2.08 | 3.92 | 2.44 | |
| MALME-3M | 2.66 | 7.88 | 2.16 | |
| M14 | 2.39 | 5.49 | 1.66 | |
| MDA-MB-435 | 1.63 | 4.44 | 1.48 | |
| SK-MEL-2 | 2.37 | 6.57 | 1.41 | |
| SK-MEL-28 | 2.35 | 5.51 | 6.55 | |
| SK-MEL-5 | 2.28 | 5.64 | 6.06 | |
| UACC-257 | 1.82 | 8.45 | 6.68 | |
| UACC-62 | 2.39 | 6.46 | 2.54 | |
| Ovarian Cancer | ||||
| IGROV1 | 2.85 | 2.60 | 3.51 | |
| OVCAR-3 | 2.20 | 5.68 | 6.52 | |
| OVCAR-4 | 2.29 | 6.59 | 1.09 | |
| OVCAR-5 | 3.68 | 13.60 | 4.5 | |
| OVCAR-8 | 2.20 | 5.91 | 0.82 | |
| NCI/ADR-RES | 2.49 | >50 | 9.43 | |
| SK-OV-3 | 2.66 | 11.70 | 3.3 | |
| Renal Cancer | ||||
| 786-0 | 2.25 | 9.42 | 1.39 | |
| A498 | 1.23 | 1.55 | 0.58 | |
| ACHN | 2.64 | 7.61 | 2.41 | |
| CAKI-1 | 2.04 | 6.80 | 2.46 | |
| RXF 393 | 1.85 | 4.11 | 0.86 | |
| SN12C | 3.35 | 5.30 | 4.52 | |
| TK-10 | 2.60 | 1.24 | 2.01 | |
| UO-31 | 2.21 | 1.80 | 3.39 | |
| Prostate Cancer | ||||
| PC-3 | 2.36 | 2.25 | 1.70 | |
| DU-145 | 3.55 | 19.10 | 2.74 | |
| Breast Cancer | ||||
| MCF7 | 2.35 | 1.75 | 3.22 | |
| MDA-MB-231/ATCC | 2.03 | 4.70 | 1.16 | |
| HS 578T | 1.57 | 2.95 | 1.23 | |
| BT-549 | 1.55 | 4.18 | 3.79 | |
| T-47D | 3.34 | 2.77 | 5.15 | |
| MDA-MB-468 | 1.67 | 1.34 | 1.05 | |
Table 1. GI50(μM) of five-dose screening results of 2, 4a and 4c.
| Subpanel tumor cell lines | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cpd. No. | Leukemia | Lung | Colon | CNS | Melanoma | Ovarian | Renal | Prostate | Breast | GI50 MG-MIDa |
| 2 | 1.97 | 2.28 | 2.84 | 2.19 | 2.22 | 2.62 | 2.27 | 2.96 | 2.09 | 2.38 |
| 4a | 4.09 | 5.93 | 5.29 | 4.88 | 6.04 | 7.68 | 4.73 | 10.68 | 2.95 | 5.81 |
| 4c | 2.96 | 1.74 | 3.42 | 1.32 | 3.44 | 4.17 | 2.2 | 2.22 | 2.6 | 2.67 |
| Sora-fenib | 1.9 | |||||||||
| a)GI50 full panel mean-graph midpoint (µM). | ||||||||||
Table 2. Median growth inhibitory concentrations (GI50, μM) of in vitro subpanel tumor cell lines and GI50 (μM) full panel mean-graph mid-points (MG-MID) of compounds 2, 4a and 4c in comparison with sorafenib.
In vitro anticancer activity
The target compounds were selected by the National Cancer Institute (NCI), USA, for anticancer activity. The screening is a two-stage process, beginning with the evaluation at a single dose (10 μM) and the compounds which display significant growth inhibition are evaluated at five concentration levels. In the first screening, the selected compounds were evaluated at a single dose (10 μM) and the culture was incubated for 48 h, utilizes 60 different human tumor cell lines. The one dose mean graphs of the selected compounds disclosed, compounds 2, 4a and 4c showed increase potency against most human cancer cell lines. So these compounds overrun to 5 dose scales (0.01–100 μM). The results are presented in tables 1-3.
| Subpanel tumor cell lines | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cpd. No. | Leukemia | Lung | Colon | CNS | Melanoma | Ovarian | Renal | Prostate | Breast |
| 2 | 1.21 | 1.05 | 0.84 | 1.09 | 1.07 | 0.9 | 1.05 | 0.81 | 1.14 |
| 4a | 1.42 | 0.98 | 1.1 | 1.19 | 0.96 | 0.76 | 1.23 | 0.54 | 1.97 |
| 4c | 0.9 | 1.53 | 0.78 | 2.03 | 0.78 | 0.64 | 1.21 | 1.2 | 1.03 |
Table 3. Selectivity ratios for compounds 2, 4a and 4c towards the nine tumor cell lines.
Touching sensitivity against individual cell lines, compound 4c showed potent anticancer activity against all human cancer cell lines with GI50 range from 0.52 to 5.15 μM. It had the highest selectivity against the cell line (SW-620) of colon cancer with GI50 0.52 μM, and against the two cell lines (HOP-92) and (HOP-62) belonging to non-small lung cancer with GI50 0.72 and 0.88 μM respectively. At the same time it showed highest activity against the two cell lines (U251) and (SNB-75) belonging to CNS cancer with GI50 0.80 and 0.83 μM respectively, and against the cell line (RXF 393) of renal cancer with GI50 0.86 μM. Compound 4a showed the highest activity against the cell line (HOP-92) belonging to non-small lung cancer with GI50 0.46 μM. The remnant of the prepared compounds offers the lowest mean percentage growth against the full 60-cell line panel. All structural modifications of ligand were performed at positions 1, 3 and 4 concerning SAR study of the target compounds via structure modifications at 4- position of 1,3,4-trisubstituted pyrazole scaffold revealed that: introduction of methylene malononitrile in 4-position of 1,3,4- trisubstituted pyrazole moiety in compound 2 proved to boost the strength towards most cancer cell lines. It has GI50 MGMID= 2.38 μM against all subpanel tumor cell lines, comparable to that of sorafenib (GI50 MG-MID=1.90 μM). Introduction of bicyclic triazolopyridine at C-4 position in it is worth mentioning that compounds 4a and 4c bearing bicyclic triazolopyridine moieties 4-position compounds 4a and 4c resulted in an improved potent anticancer activity.
4-Tolyl-triazolopyridine 4c (GI50 MG-MID=2.67 μM) showed almost equipotent GI50 value to that expressed by methylene malononitrile derivative 2. 2-Furyl triazolopyridine 4a displayed two-fold decrease in anticancer activity (GI50 MGMID= 5.81 μM) than its 4-tolyl counterpart 4c. In addition, 2- Furyl triazolopyridine 4a displayed two-fold reduction in anticancer activity (GI50 MG-MID = 5.81 μM) than its 4-tolyl counterpart 4c. In particular, compound 6 bearing a tricyclic indenopyridine did not show marked anticancer activity.
Conclusions
The objective of the present study was to evaluate the potential anticancer activities of some pyrazole compounds attached to bicyclic and tricyclic ring systems. The bicyclic derivative 4c showed potent anticancer activity against majority human tumor cell lines with GI50 0.52-5.15 μM, and could be considered as promising selective anticancer lead for further development of more potent anticancer agents.
Acknowledgements
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
References
- Khan Z, Bisen P. Oncoapoptoticsignaling and deregulated target genes in cancers: special reference to oral cancer. Biochem. Biophys. Acta 2013; 1836: 123-145.
- Nersesyan H, Slavin K. Current aproach to cancer pain management: Availability and implications of different treatment options. TherClin Risk Manag 2007; 3: 381-400
- Barreiro EJ. Medicinal Chemistry and the Paradigm of the Lead Compound. Rev. Virtual Quim 2009; 1: 26-34.
- Wilson WL, Bottiglieri NG. Phase I studies with pyrazole. Cancer Chemother Rep 1962; 21: 137-141.
- Xia Y, Dong ZW, Zhao BX, Ge X, Meng N, Shin D, Miao JY. Synthesis and structure-activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives as potential agents against A549 lung cancer cells. Bioorg Med Chem 2007; 15: 6893-6899.
- Daidone G, Maggio B, Raffa D, Plescia S, Schillaci D, Raimondi MV. Synthesis and in vitro antileukemic activity of new 4-triazenopyrazole derivatives. Farmaco 2004; 59: 413-417.
- Farghaly AR. Synthesis of some new indole derivatives containing pyrazoles with potential antitumor activity. ARKIVOC 2010; 11: 177-187.
- Soliman EA, El-Zahar MI, El-Masry AH, Kamel M, Gohar RS. Synthesis and anticancer evaluation of novel tetrahydronaphthalen-6-ylthiazole heterocycles against human HePG2 and MCF7 cell lines. Der PharmaChem 2010; 2: 507-521.
- El-Zahar MI, EL-Karim SA, Haiba ME, Khedr MA. Synthesis, antitumor activity and molecular docking study of novel benzofuran-2-yl pyrazole pyrimidine derivatives. Acta Pol Pharm Drug Res 2011; 68: 357-373.
- Kalirajan R, Rathore L, Jubie S, Gowramma B, Gomathy S, Sankar S. Microwave assisted synthesis of some novel pyrazole substituted benzimidazoles and evaluation of their biological activities. Indian J Chem 2011; 50B: 1794-1799.
- Kasiotis KM, Tzanetou EN, Haroutounian SA. Pyrazoles as potential anti-angiogenesis agents: a contemporary overview. Front. Chem 2014; 2: 78-85.
- Perchellet EM, Ward MM, Skaltsounis AL, Kostakis IK, Pouli N, Marakos P, Perchellet JH. Antiproliferative and proapoptotic activities of pyranoxanthenones, pyranothioxanthenones and their pyrazole-fused derivatives in HL-60 cells. Anticancer Res 2006; 26: 2791-2804.
- Insuasty B, Tigreros A, Orozco F, Quiroga J, Abonia R, Nogueras M, Sanchez A, Cobo J. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem 2010; 18: 4965-4974.
- Labbozzetta M, Baruchello R, Marchetti P, Gueli MC, Poma P, Notarbartolo M, Simoni D, D’Alessandro N. Lack of nucleophilic addition in the isoxazole and pyrazolediketone modified analogs of curcumin; implications for their antitumor and chemosensitizing activities. Chem-Biol Interact 2009; 181: 29-36.
- Eweas AF, Khalifa NM, Ismail N, Al-Omar MA, Soliman AM. Synthesis, Docking and Biological Activities of Novel Hybrids Celecoxib and AnthraquinoneAnalogs as Potent Cytotoxic Agents. Med Chem Res 2014; 23: 76-86.
- Almutairi MS, Hegazy GH, Haiba ME, Ali HI, Khalifa NM, Soliman AM. Synthesis, Docking and Biological Activities of Novel Hybrids Celecoxib and AnthraquinoneAnalogs as Potent Cytotoxic Agents. Int J MolSci 2014; 15: 22580-22603.
- Haiba ME, Al-Abdullah ES, Edrees MM, Khalifa NM. Synthesis, Docking and Biological Activities of Novel Hybrids Celecoxib and AnthraquinoneAnalogs as Potent Cytotoxic Agents. Drug Res 2015; 65: 9-17.
- Alley MC, Scudiero DA, Monks PA, Hursey ML, Fine MJ, Czerwinski DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculturetetrazolium assay. Cancer Res 1988; 48: 589-601.
- Grever MR, Schepartz SA, Chabner BA. discovery and development program. SeminOncol 1992; 19: 622-638.
- Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res 1995; 34: 91-109.