- Biomedical Research (2016) Volume 27, Issue 3
Anticancer activities of some synthesized 2,4,6-trisubstituted pyridine candidates.
Abd El-Galil E Amr1,2, Mohamed M Abdalla3*1Pharmaceutical Chemistry Department, Drug Exploration & Development Chair (DEDC), College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia
2Applied Chemistry Department National Research Center, 12622-Dokki, Cairo, Egypt
3Research Unit, Saco Pharm. Co., 6th October City 11632, Egypt
Accepted date: February 25, 2016
Abstract
We herein report the anti-cancer activities of some synthesized pyridine derivatives substituted with thiazole, pyrazole and triazole moieties. A series of these derivatives 1-12 were synthesized and evaluated as anti-inflammatory, analgesic, anticonvulsant and antiparkinsonian agents before. Twelve compounds were conveniently screened for their in vitro cytoyoxicity against a wide rannge of cell lines and they are also showed potent activities against renal and prostate cancer cell lines. The in vivo antirenal cancer and antiprostate cancer of the most active in vitro compounds was estimated and founded highly potent. In search for the mechanism of action of anticancer activities it was foundeded that these compounds exert its action via histone decarboylase inhibition and inhibition of p53 ubiquitination.
Keywords
2,4,6-Trisubstituted pyridine, Heterocyclic derivatives, Anticancer activities.
Abbreviations
ARF: Alternative Reading Frame; DMEM: Dulbecco's Modified Eagle's Medium; DMSO: Dimethyl Sulfoxide; HLI98: HDM2 Ligase Inhibitor 98 Class.
Introduction
Thieno[2,3-b]pyridine candidates which substituted with pyridine, cyclopentyl, tetrahydroquinoline, pyrimidine, 1,6- naphthiridin, benzofuro[2,3-b]pyridine, imidazo[1,2- c]pyrimidine, triazolo[1,5-a]pyrimidine were synthesized and used a new agents for their in vitro antitumor activities against liver HepG-2 and breast MCF-7 cell lines [1-3]. Many pyridine derivatives were synthesised and foundeded to have cytotoxic activities in vitro against a wide range of cell lines especially renal and prostate types [4]. Imidazopyridine derivatives have potent antitumor activities [5]. In addition, two series of 4,6- diaryl-2-imino-1,2-dihydropyridine-3-carbonitriles and imidazo[2,1-b]pyridine/ pyrimidine chalcones were also synthesized and evaluated for their in vitro capacity to inhibit PDE3A and the growth of the human HT-29 colon adenocarcinoma tumor cell line [6-8]. In view of these observations and in continuation of our previous work [9-16] in biological and pharmacological studies for heterocyclic candidates, we herein reported the anti-cancer activities of some heterocyclic substituted pyridine derivatives.
Experimental
Chemistry
All the tested compounds 1-12 were confirmed by physical and spectroscopic evidences according to the previously reported procedure [16].
Cytotoxic activities
In vitro determination of IC50 of tested compounds against differrent cancer cell line via using the MTT Assay
The cytotoxicity of the newly synthesized compounds against cancer cell lines in vitro was performed with the MTT assay according to the previous reported method [17].
In vivo determination of the cytotoxic activities
Effects of compounds tested compounds on prostate cancer in vivo
To investigate the effect of tested compounds on prostate cancer cells in vivo use the adopted methods [18,19].
In vivo antirenal cancer
Cell lines: OUR-10 cells were maintained in RPMI-1640 medium containing 10% heat-inactivated fetal calf serum (FCS). OUR-10 and DU145 cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated FCS.
Proliferation assay: The experimental method which was used in proliferation assay has been adopted from Oka et al. [20].
Xenograft model: The experimental method which was used in xenograft model has been adopted from Liang et al. [21].
In vitro inhibition of histone deacetylase: The experimental method which was used in vitro inhibition of histone deacetylase has been adopted from Yoshid et al., [22] and Farooq et al., [23].
Biological assay in vitro ubiquitination assay: The experimental method which was used for biological assay in vitro ubiquitination assay has been adopted from Roxburgh et al., [24].
Non-fluorescent in vitro ubiquitination assays: The experimental method which was used for non-fluorescent in vitro ubiquitination assays has been adopted from Roxburgh et al., [24].
Statistical analysis
The data were expressed as means plus; standard deviation (s.d.). Statistical analysis was performed by Student's t-test (two-tailed). The criterion for statistical significance was taken as P<0.05.
Results and Discussion
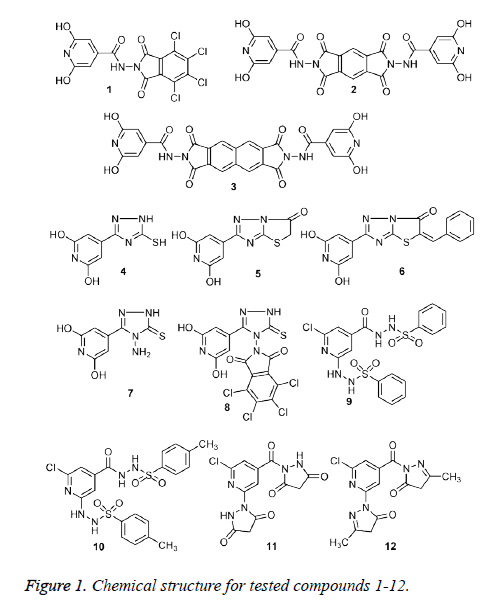
In continuation of our previous work, a series of substituted pyridine, pyrazole, triazole and thiazolotriazole derivatives 1-12 (Figure 1) were synthesized before and screened as anti-inflammatory, analgesic, anticonvulsant and antiparkinsonian agents [16]. Herein, we report the activities of these compounds for evaluation as anticancer agents.
Anti-cancer activities
The cytotoxicity of the newly synthesized compounds against cancer cell lines in vitro was performed with the MTT assay according to the previous reported [17].
All the tested compounds showed potent cytotoxic activities in vitro against aKB6, SKOV-3, SF-268, NCI H 460, RKOP27, PC3, OUR-10, HL60, U937, K561, G361, SK-MEL-28, GOTO, NB-1, HeLa, MCF-7, HT1080 and HepG2 at micro molar level for all cell except for PC3, OUR-10 the potent activities lies on nanomolar level (Table 1).
| Comp. No. | IC50 µ M Tumor cell growth inhibition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KB6 | SK OV-3 | SF-268 | NCI H 460 | RKOP27 | PC3 | OUR-10 | |||||
| 1 | 0.29 | 0.98 | 0.88 | 0.067 | 0.03 | 0.00057 | 0.000099 | ||||
| 2 | 0.28 | 0.97 | 0.87 | 0.066 | 0.044 | 0.00055 | 0.000091 | ||||
| 3 | 0.27 | 0.96 | 0.86 | 0.055 | 0.055 | 0.00053 | 0.000086 | ||||
| 4 | 0.26 | 0.88 | 0.85 | 0.054 | 0.046 | 0.00051 | 0.000084 | ||||
| 5 | 0.25 | 0.86 | 0.74 | 0.053 | 0.063 | 0.00049 | 0.000079 | ||||
| 6 | 0.24 | 0.84 | 0.67 | 0.052 | 0.052 | 0.00048 | 0.000073 | ||||
| 7 | 0.23 | 0.83 | 0.58 | 0.047 | 0.043 | 0.00046 | 0.000068 | ||||
| 8 | 0.21 | 0.76 | 0.48 | 0.038 | 0.054 | 0.00043 | 0.000066 | ||||
| 9 | 0.14 | 0.56 | 0.36 | 0.026 | 0.022 | 0.00034 | 0.000052 | ||||
| 10 | 0.16 | 0.67 | 0.38 | 0.037 | 0.013 | 0.00035 | 0.000054 | ||||
| 11 | 0.13 | 0.55 | 0.35 | 0.024 | 0.033 | 0.00023 | 0.000044 | ||||
| 12 | 0.12 | 0.54 | 0.33 | 0.021 | 0.032 | 0.00021 | 0.000043 | ||||
| Comp. No. | Leukemia | Melanoma | Neuro-blastoma | Cervical | Breast | Fibrosa-rcoma | liver | ||||
| HL60 | U937 | K561 | G361 | SK-MEL-28 | GOTO | NB-1 | HeLa | MCF-7 | HT1080 | Hep-G2 | |
| 1 | 0.88 | 0.87 | 0.35 | 0.56 | 0.67 | 0.56 | 0.45 | 0.34 | 0.45 | 0.56 | 0.23 |
| 2 | 0.77 | 0.95 | 0.46 | 0.67 | 0.76 | 0.65 | 0.42 | 0.25 | 0.46 | 0.34 | 0.67 |
| 3 | 0.64 | 0.06 | 0.57 | 0.75 | 0.84 | 0.73 | 0.57 | 0.13 | 0.55 | 0.12 | 0.45 |
| 4 | 0.55 | 0.95 | 0.65 | 0.84 | 0.93 | 0.47 | 0.43 | 0.96 | 0.64 | 0.32 | 0.43 |
| 5 | 0.63 | 0.84 | 0.74 | 0.93 | 0.82 | 0.46 | 0.67 | 0.87 | 0.75 | 0.45 | 0.75 |
| 6 | 0.34 | 0.73 | 0.85 | 0.82 | 0.76 | 0.68 | 0.85 | 0.78 | 0.86 | 0.32 | 0.98 |
| 7 | 0.47 | 0.52 | 0.76 | 0.76 | 0.65 | 0.59 | 0.77 | 0.59 | 0.75 | 0.68 | 0.78 |
| 8 | 0.56 | 0.66 | 0.67 | 0.67 | 0.58 | 0.44 | 0.68 | 0.67 | 0.66 | 0.45 | 0.66 |
| 9 | 0.26 | 0.48 | 0.37 | 0.54 | 0.29 | 0.16 | 0.59 | 0.55 | 0.34 | 0.67 | 0.24 |
| 10 | 0.47 | 0.37 | 0.28 | 0.45 | 0.47 | 0.35 | 0.48 | 0.36 | 0.25 | 0.63 | 0.57 |
| 11 | 0.35 | 0.27 | 0.46 | 0.35 | 0.30 | 0.26 | 0.30 | 0.44 | 0.43 | 0.54 | 0.43 |
| 12 | 0.24 | 0.35 | 0.55 | 0.26 | 0.19 | 0.37 | 0.29 | 0.35 | 0.14 | 0.24 | 0.12 |
Table 1: In vitro cytotoxicity activities of the tested compounds 1-12 against several cancer cell lines using MTT assay.
In vivo antiprostate cancer
The in vivo anti-prostate cancer activities of the tested compounds were estimated and culminated on the tested compounds exerts potent anti-prostate carcinoma in the following descending order 12, 11, 9, 10, 8, 7, 6, 5, 4, 3, 2 and 1 (Table 2).
| Comp. No. | % Decrease in PC3 tumor volume |
|---|---|
| Control | 230 |
| 1 | 53 |
| 2 | 46 |
| 3 | 45 |
| 4 | 42 |
| 5 | 38 |
| 6 | 36 |
| 7 | 31 |
| 8 | 29 |
| 9 | 27 |
| 10 | 28 |
| 11 | 25 |
| 12 | 22 |
Table 2: In vivo antiprostate carcinoma for tested compounds 1-12.
In vivo anti-renal cancer
In this study, from table 3 compounds 1-12 were used to test the effect on renal cancer cell lines. As shown in table 3, treatment with tested compounds reduced cell viability of renal cancer cells (OUR-10) in a time-dependent manner [18].
| Comp. No. | %Cell Viability after time for cell line OUR-10 | ||
|---|---|---|---|
| Day 1 | 3 Days | 5 Days | |
| 1 | 38 | 25 | 12 |
| 2 | 34 | 22 | 11 |
| 3 | 31 | 21 | 10 |
| 4 | 29 | 19 | 9 |
| 5 | 28 | 17 | 8 |
| 6 | 27 | 16 | 8 |
| 7 | 25 | 15 | 7 |
| 8 | 24 | 13 | 7 |
| 9 | 18 | 10 | 6 |
| 10 | 22 | 11 | 6 |
| 11 | 14 | 9 | 5 |
| 12 | 12 | 8 | 5 |
Table 3: Cytotoxic effect of treatment with tested compounds 1-12 against OUR-10.
Xenograft model
We examined whether tested compounds could inhibit the growth of OUR-10 tumors in mice. We selected an injection dose of 7 μM/Kg 3 times per week because this dose effectively inhibited in vivo OUR-10 tumor growth without adverse effects in a preliminary study. About 6 weeks after the start of treatment with compounds 12, 11, 9, 10, 8, 7, 6, 5, 4, 3, 2 and 1, the increase in OUR-10 tumor volume was measured and given in table 4. The in vivo antirenal cancer activities of the tested compounds were estimated and culminated on the tested compounds exerts potent anti prostate carcinoma in the following descending order 12, 11, 9, 10, 8, 7, 6, 5, 4, 3, 2 and 1.
| Comp. No. | Tumor volume mm3 |
|---|---|
| Control | 988 |
| 1 | 291 |
| 2 | 288 |
| 3 | 278 |
| 4 | 255 |
| 5 | 243 |
| 6 | 238 |
| 7 | 224 |
| 8 | 212 |
| 9 | 167 |
| 10 | 181 |
| 11 | 155 |
| 12 | 138 |
Table 4: In vivo antirenal carcinoma for tested compounds 1-12.
Mechanism of anticancer activities
In search for the mechanism of action of the anticancer activities of the tested compounds the author's resorts to many in vitro assays for many targeted enzymes and cancer modulation molecules, these experiments revealed on the following two mechanistic pathways.
In vitro Inhibition of histone deacetylase
All the tested compounds inhibited the enzyme histone decarboxylase in the following descending order 12, 11, 9, 10, 8, 7, 6, 5, 4, 3, 2 and 1 (Table 5).
| Comp. No. | IC50 (nM) |
|---|---|
| 1 | 0.0066 |
| 2 | 0.0059 |
| 3 | 0.0058 |
| 4 | 0.0057 |
| 5 | 0.0056 |
| 6 | 0.0055 |
| 7 | 0.0054 |
| 8 | 0.0047 |
| 9 | 0.0045 |
| 10 | 0.0046 |
| 11 | 0.0044 |
| 12 | 0.0043 |
Table 5: IC50 of histone decarboxylate inhibitor activities of the tested compounds1-12.
Ubiquitination assay
All the testes compounds showed inhibition of p53 ubiquitination, the most active ones were 12, 11, 9, 10, 8, 7, 6, 5, 4, 3, 2 and 1 (Table 6) [18].
| Comp. No. | IC50 of p53 ubiquitination |
|---|---|
| 1 | 0.77 |
| 2 | 0.71 |
| 3 | 0.69 |
| 4 | 0.67 |
| 5 | 0.57 |
| 6 | 0.55 |
| 7 | 0.53 |
| 8 | 0.44 |
| 9 | 0.48 |
| 10 | 0.42 |
| 11 | 0.33 |
| 12 | 0.31 |
| Diph. Imidazole | 0.26 (17) |
Table 6: IC50 of p53 ubiquitination of the newly synthesized compounds 1-12 (in vitro).
Conclusion
All tested compounds inhibited in vivo prostate and renal carcinoma via inhibition of the histone decaroxylase and inhibition of p53 ubiquitination also inhibited the EGFR and VEGFR-2 kinase.
Structural Activity Relationships
• Chloropyridine and pyrazoles essential for potent activities but the methyl pyrazole exerts more potent activities than the oxo-ones, while sulfones showed the least potent activities.
• Hydroxy pyridines provide less potent activities than the chloropyridines.
• Fusion of another heterocyclic ring system into the triazole ring greatly reduces the activities.
• Macrocyclic moieties and polychloro ones greatly reduces the activities.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group project no. RGP -172.
References
- Wu JP, Fleck R, Brickwood J, Capolino A, Catron K, Chen Z, Cywin C, Emeigh J, Foerst M, Ginn J, Hrapchak M, Hickey E, Hao MH, Kashem M, Li J, Liu W,Morwick T, Nelson R, Marshall D, Martin L, Nemoto P, Potocki I, Liuzzi M, Peet GW, Scouten E, Stefany D, Turner M, Weldon S, Zimmitti C, Spero D, Kelly TA. The discovery of thienopyridine analogues as potent IkappaB kinase beta inhibitors. Part II. Bioorg Med Chem Lett 2009; 19: 5547-5551.
- Willemann C, Grünert R, Bednarski PJ, Troschütz R. Synthesis and cytotoxic activity of 5,6-heteroaromatically annulated pyridine-2,4-diamines. Bioorg Med Chem 2009; 17: 4406-4419.
- Lockman J W, Reeder M D, Suzuki K, Ostanin K, Hoff R, Bhoite L, Austin H, Baichwal V, Adam Willardsen J. Inhibition of eEF2-K by thieno[2,3-b]pyridine analogues. Bioorg. Med Chem Lett 2010; 20: 2283-2286.
- Hammam AG, Sharaf MA, Abdel Hafez NA. Synthesis and anti-cancer activity of pyridineand thiazolopyrimidine derivatives using1-ethylpiperidone as a synthon. Indian J Chem 2001; 40B: 213-221.
- Muniyan S, Cho YW, Ingersoll MA, Devine A, Morris M, Odero-Marah VA, Khan SA, Chaney WG, Bu XR, Lin MF. Antiproliferative activity of novel imidazopyridine derivatives on castration-resistant human prostate cancer cells. Cancer Letters 2014; 353: 59-67.
- Abadi AH, Ibrahim TM, Abouzid KM, Lehmann J, Tinsley HN, Gary BD, Piazza GA. Design, synthesis and biological evaluation of novel pyridine derivatives as anticancer agents and phosphodiesterase 3 inhibitors. Bioorg Med Chem 2009; 17: 5974-5982.
- Kamal A, Reddy JS, Ramaiah MJ, Dastagiri D, Bharathi EV, Sagar MVP, Pushpavalli S, Ray P, Pal-Bhadra M. Design, synthesis and biological evaluation of imidazopyridine/pyrimidine-chalconederivatives as potential anticancer agents. Med Chem Commun 2010; 1: 355-362.
- Zhao X, Huang W, Wang Y, Xin M, Jin Q, Cai J, Tang F, Zhao Y, Xiang H. Pyrrolo[2,3-b]pyridine derivatives as potent Bruton's tyrosine kinase inhibitors. Bioorg Med Chem 2015; 23: 4344-4353.
- Abdalla MM, Al-Omar MA, Al-Salahi RA, Amr AE, Sabry NM. A new investigation for some steroidal derivatives as anti-alzheimer agents. Int J Biol Macromol 2012; 51: 56-63.
- Amr AE, Mohamed AM, Mohamed SF, Abdel-Hafez NA, Hammam AG. Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg Med Chem 2006; 14: 5481-5488.
- Abdel Salam OI, Khalifa NM, Said SA, Amr AE. Synthesis and antimicrobial activities of some newly 2,4,6-tri-substituted pyridine derivatives. Res Chem Intermed 2014; 40: 1147-1155.
- Flefel EM, Tantawy WA, Abdel-Mageid RE, Amr AE, Nadeem R. Synthesis and anti-viral activities of some 3-(naphthalen-1-ylmethylene)-5-phenylfuran-2(3H)-one candidates. Res Chem Intermed 2015; 40:1365-1381
- Ouf NH, Amr AE, Sakran MI. Anticancer activity of some newly synthesized pyrano[2,3-d][1,2,3]triazine derivatives using 1-(7-hydroxy-2,2-dimethyl-chroman-6-yl)ethanone as synthon. Med Chem Res 2015; 24: 1514-1526.
- Said SA, El-Sayed HA, Amr AE, Abdalla MM. Selective and orally bioavailable CHK1 inhibitors of some synthesized substituted thieno[2,3-b]pyridine candidates. Int. J. Pharmacol 2015; 11: 659-671.
- Hussain AA, Abdulla MM, Amr AE, Al-Omar MA, Shalaby AFA. Anti-inflammatory activities of some newly synthesized pyridinyl- and indazolyl benzamide derivatives. Russ J Bioorg Chem 2015; 41: 87-96.
- Al-Omar MA, Amr AE, Al-Salahi RA. Anti-inflammatory, analgesic, anticonvulsant and antiparkinsonian activities of some pyridine derivatives using 2,6-di-substituted isonicotinic acid hydrazides. Arch. Pharm. Chem. Life Sci 2010; 10: 648-656.
- McCauley J, Zivanovic A, Skropeta D. Bioassays for anticancer activities. Methods in Molecular Biology 2013; 1055: 191-205.
- Kondo S, Kondo Y, Li G, Silverman RH, Cowell JK. Targeted therapy of human malignant glioma in a mouse model by 2-5A antisense directed against telomerase RNA. Oncogene 1998; 16: 3323-3330.
- Kondo Y, Koga S, Komata T, Kondo S. Treatment of prostate cancer in vitro and in vivo with 2-5A-anti-telomerase RNA component. Oncogene 2000; 19: 2205-2211.
- Oka D, Nishimura K, Shiba M, Nakai Y, Arai Y, Nakayama M, Takayama H, Inoue H, Okuyama A, Nonomura N. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-kappaB. Int J Cancer 2007; 120: 2576-2581.
- Liang XI, Zhang JO, Liu ZC, Zhang JH, Yan JF, Jin Y, Lin J. Novel 5-anilinoquina-zoline-8-nitro derivatives as inhibitors of VEGFR-2 tyrosine kinase: synthesis, biological evaluation and molecular docking. Org Biomol Chem 2013; 11: 4367-4378.
- Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A J Biol Chem 1990; 265: 17174-17179.
- Farooq M, Hozzein WH, Elsayed EA, Taha N, Wadaan MA. Identification of histone deacetylase 1 protein complexes in liver cancer cells. Asian Pacific J. Cancer Preven 2013;14: 915-921.
- Roxburgh P, Hock AK, Dickens MP, Mezna M, Fischer P M, Vousden KH. Small molecules that bind the Mdm2 RING stabilize and activate p53. Carcinogenesis 2012; 33: 791-798.