Research Article - Biomedical Research (2017) Volume 28, Issue 4
Antibacterial activity and morphological changes in human pathogenic bacteria caused by Chlorella vulgaris extracts
Hend Alwathnani, Kahkashan Perveen*Department of Botany and Microbiology, College of Science, King Saud University, Riyadh-11495, Kingdom of Saudi Arabia
- *Corresponding Author:
- Kahkashan Perveen
Department of Botany and Microbiology
College of Science King Saud University, Saudi Arabia
Accepted on August 25, 2016
Abstract
Extracts of Chlorella vulgaris prepared by using three solvent; methanol, chloroform and diethyl ether, were evaluated for the antimicrobial potential. The extracts were tested against Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25928, Streptococcus pyogenes grp A (clinical isolate), Bacillus subtilis ATCC 6633. The findings revealed that the extracts were able to inhibit the growth of one or more tested pathogen. The diethyl ether extract showed the maximum zone of inhibition (28.6 mm) against E. coli whereas these extracts were ineffective against P. aeruginosa and S. aureus. The MIC of diethyl ether extract was 0.6 mg/ml against E. coli and S. pyogenes whereas, the MIC of acetone extract was 1.0 mg/ml for B. subtilis. The scanning electron microscopy (SEM) was used to examine the morphological changes in E. coli induced by diethyl ether extract of C. vulgaris. The treated E. coli cells were deformed, their surfaces were rough and depressions in some cells were also observed. The GC-MS analysis of extract detected the presence of Heptanal, a known biomedical compound. The other major components of the extract were 2-Butanol, 3-Methyl-, (S)-; 2-Hexanol, (S)-; (3R, 2E)-2-(Hexadec-15-Ynyliedene)-3-Hydroxy-4-Methylenebutanolide; 4- Methyldocosane. Thus, it can be concluded that extracts of C. vulgaris, have the potential to be explored for antibiotic production. Further, efforts should be made to identify the compounds directly responsible for antibacterial properties.
Keywords
Antimicrobial activity, Human pathogenic bacteria, Minimum inhibitory concentration, SEM.
Introduction
The problem of microbial resistance to antibiotics is increasing with time. The indiscriminate use of antibiotics for the treatment of diseases is one of the reasons of development of multidrug resistant pathogens. Till now microbial resistance to almost all antibiotics has been reported, additionally the side effects associated with antibiotics has increased the problem [1,2]. Therefore, there is a need to discover the new spectrum of antimicrobial agents which have minimal side effects. Chlorella is a green microalga; it is widely used in pharmaceutical and cosmetic industries. Chlorella has high nutritional values; therefore, it is also used in food and health supplements [3]. Several workers have reported antimicrobial activity of extracts of Chlorella. However, the morphological changes in pathogenic bacteria due to these extracts have not been explored [4,5]. Moreover, there is a continuous need for new chemicals which can be effective against microorganisms and serve as safer antibiotics. Therefore, an investigation was carried out to evaluate the bioactivity of extracts of C. vulgaris against some important human pathogenic bacteria. The morphological changes in sensitive bacteria were observed by Scanning Electron Microscopy (SEM) and the chemical composition of the bioactive extract was determined by GCMS analysis.
Materials and Methods
Microorganisms
Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25928, Streptococcus pyogenes grp A (clinical isolate) and Bacillus subtilis ATCC 6633, were procured from the Department of Botany and Microbiology, King Saud University. Stock cultures of bacterial strains were maintained on the slants of Mueller Hinton Agar (MHA), all stock cultures were stored at 4°C.
Cultivation of Chlorella vulgaris
The culture of C. vulgaris was obtained from the Department of Botany and Microbiology, King Saud University. The microalgae were subcultured in BG 11 nutrition media and the culture was allowed to flourish for 2-4 weeks at 20-30°C under constant light [6]. Cells of the active culture were harvested by filtering through Whatman no. 1 filter paper and the extracted biomass was used for the preparation of crude extract.
Preparation of the crude extract
Five-gram biomass of C. vulgaris was mixed with 100 ml of acetone in a glass flask and was kept for 3 days at 20°C on a shaker. The solution was filtered through Whatman no. 1 filter paper. The same methodology was applied to the solvent diethyl ether and methanol. Extracts were kept under the fume hood in a water bath at 40°C and extracts were evaporated to dryness. The obtained residue was dissolved in the respective solvent to get the final concentration of 50 mg/ml of the crude extract.
Determination of antibacterial activity by agar well diffusion method
Antibacterial assay: The antibacterial activity of crude extracts against five human pathogens was determined by agar well diffusion assay [7]. In this method, 10 ml aliquots of Mueller Hinton Broth (MHB) was inoculated with the bacterial pathogens and incubated at 37°C for 24 h. After 24 h the population of bacteria was adjusted to 1 × 106 CFU/ml and 100 μl bacterial suspension was spread evenly with sterile spreader over the entire surface of the MHA plate to obtain uniform inoculum. In each agar plate 4 wells were made with the reverse side of the sterilized micropipette tips. The crude extract of C. vulgaris (50 μl/well) was poured into respective wells. Ampicillin and the respective solvents were used as the positive and the negative control respectively. The plates were incubated for 24 h at 37°C. The experiment was carried out in triplicates. After 24 h the diameter of zone of inhibition around the well was measured (mm).
Determination of minimum inhibitory concentration (MIC)
Minimum Inhibitory Concentration (MIC) was determined by the streak method [8]. To determine the MIC, extracts were dissolved in MHB and serially diluted in eppendorf tubes under a laminar flow cabinet. The uniform volume of an actively growing culture of the tested pathogen was added to the different eppendorf tubes and cultures were grown overnight in an incubator at 37°C. The following morning, streaking was done from all samples on respective agar plates. MIC was rated by the lowest concentration of the test solution that inhibited growth.
Preparation of cells for SEM
On the basis of the results of antibacterial activity of C. vulgaris extracts, the most sensitive bacterial strain was selected for observing the morphological changes in cells. The microscopy was performed with JSM-6060LV Scanning Electron Microscope (SEM). The fresh bacteria culture (in MHB) was treated with the sub-MIC of crude extract whereas, the bacterial culture without crude extract served as untreated controls. Both cultures were incubated for 24 h at 37°C. After incubation, 10 μl of treated bacteria were fixed in 3% glutaraldehyde buffered with 0.1 M sodium phosphate buffer (pH 7.2) for an hour then washed and post-fixed in 1% osmium tetroxide again for one hour. After washing with 0.15 M sodium phosphate buffer (pH 7.2) the samples were dehydrated in a graded alcohol series. The last stage of dehydration was performed with propylene oxide. The specimens were dried and mounted onto stubs using double-sided carbon tape after that they were coated with a thin layer of gold. The prepared specimens were observed under SEM at 120-keV electron energy.
GC-MS analysis of crude extracts
The crude extract which showed strong positive antimicrobial activity was analyzed for its chemical composition. The analysis was done by using Perkin Elmer (Clarus 500, USA) gas chromatography coupled with (Clarus 500, USA) mass spectrometer (MS) equipped with RTx-5 column (30 × 0.32 nm). The oven temperature was initially held at 75°C for 2 min, then increased to 75 to 175°C at a rate of 50°C per min and finally held at 175°C for 7 min. Helium (3 ml/min at constant flow) was used as a carrier gas. In this chromatographic analysis, neither internal nor external chemical standards were used. The interpretation of the resultant mass spectra was made with the help of a computerized library-searching program (NIST database) and by analyzing the fragmentation pattern of the compound obtained from mass spectrometry analysis.
Results
The antimicrobial activity of extracts of C. vulgaris prepared by using methanol, chloroform and diethyl ether solvents was recorded in Table 1. Data clearly show the great potency of C. vulgaris extracts to inhibit the bacterial growth. All extracts were able to inhibit the growth of one or more pathogen and the zone of inhibition ranges from 17.4 to 28.6 mm whereas, these extracts were ineffective against P. aeruginosa and S.aureus. Diethyl ether solvent extract was found to be more effective than other extracts of C. vulgaris. The maximum zone of inhibition by the diethyl ether extract was observed against E. coli (28.6 mm), while acetone and methanol, extracts showed the maximum zone of inhibition against B. subtilis (27.2 and 25.6 mm respectively). The results of determination of minimum inhibitory concentration (MIC) conducted against sensitive bacteria showed that the MIC of the diethyl ether extract was 0.6 mg/ml against E. coli and S. pyogenes whereas MIC of acetone extract against B. subtilis was 1.0 mg/ml (Table 1).
| Organisms | Extracts (50 µl/well) | Positive control* | MIC (mg/ml) | ||
|---|---|---|---|---|---|
| Acetone | Methanol | Diethyl ether | |||
| Zone of inhibition (mm) a | |||||
| P. aeruginosa | 0 | 0 | 0 | 30.3 ± 0.58 | - |
| E. coli | 26.8 ± 0.29 | 25.2 ± 0.21 | 28.6 ± 0.58 | 31.8 ± 0.58 | 0.6 Diethyl ether |
| S. aureus | 0 | 0 | 0 | 23.0 ± 0.58 | - |
| S. pyogenes | 17.4 ± 0.21 | 18.4 ± 0.36 | 19.1 ± 0.21 | 22.3 ± 0.58 | 0.6 Diethyl ether |
| B. subtilis | 27.2 ± 0.35 | 25.6 ± 0.51 | 0 | 30.0 ± 1.15 | 1.0 Acetone |
amean ±SD for N=3; *antibiotic: Ampicillin 40 μg/ml; negative control did not show any zone of inhibition.
Table 1. Antibacterial activity of C. vulgaris extracts against human pathogenic bacteria evaluated by well diffusion assay.
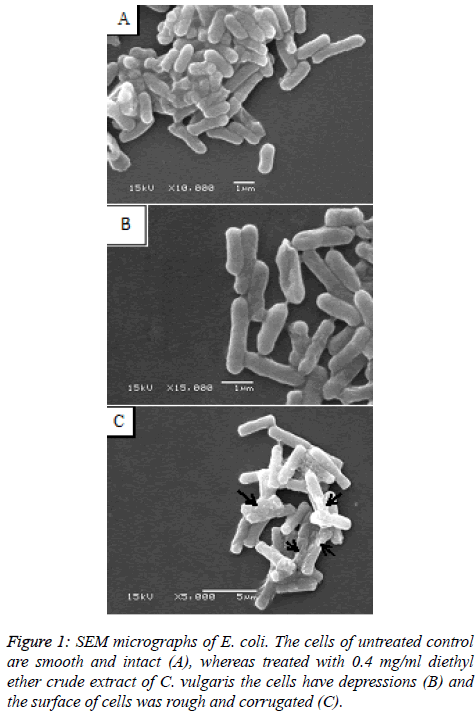
As the diethyl ether extract was found to be the most effective for the growth inhibition of E. coli, the morphological changes were studied by SEM for clearer understanding of the action. The E. coli cells treated with sub-MIC of the diethyl ether crude extract of C. vulgaris were deformed and shrunken while the surfaces of some cells were rough with depressions (Figures 1B and 1C), against the untreated E. coli cells which displayed a smooth and intact surface (Figure 1A). The result of GC-MS analysis of crude extract of C. vulgaris presented in Table 2 revealed that the major components of the extract were 2-Butanol, 3-Methyl-, (S)-; 2-Hexanol, (S)-; (3R, 2E)-2- (Hexadec-15-Ynyliedene)-3-Hydroxy-4-Methylenebutanolide; Heptanal; 4-Methyldocosane beside that several commonly known esters, polysaccharides were also identified.
| Compound | Molecular formula (M.F) | Molecular weight (MW) (g/mol) | Rev |
|---|---|---|---|
| 2-Butanol, 3-Methyl-, (S)- | C5H12O | 88 | 908 |
| 2-Hexanol, (S)- | C6H14O | 102 | 905 |
| 2(3H)-Furanone, 3-(15-Hexadecynylidene)Dihydro-4-Hydroxy-5-Methyl- | C21H34O3 | 334 | 887 |
| 3-Methyl-2-(3-Methylpentyl)-3-Buten-1-Ol | C11H22O | 170 | 884 |
| 4-Methyldocosane | C23H48 | 324 | 879 |
| N-(3-MethylButyl) Acetamide | C7H15NO | 129 | 776 |
| 5,9-Dodecadien-2-one,6,10-Dimethyl-, (E,E)- | C14H24O | 208 | 773 |
| Heptanal | C7H14O | 114 | 772 |
| D-Mannoheptadecane-1,2,3,4,5-Pentaol (methyl ester-fatty acid) | C17H36O5 | 320 | 769 |
| 3-Decyn-2-Ol | C10H18O | 154 | 763 |
Table 2. The GC-MS analysis of crude extract of C. vulgaris.
Discussion
Development of microbial resistance against known antibiotic is of great concern to the world of medicine. There is a serious urge for searching the alternative source of antibiotics which can provide cure to diseases caused by pathogenic microbes. Extracts of various cyanobacteria have been explored for the antibacterial activity [9,10]. In the current research methanol, acetone and diethyl ether solvents were used to prepare the extracts of C. vulgaris. The extracts were evaluated for five human pathogenic bacteria. Diethyl ether extract was found to be most effective in inhibiting the growth of E. coli while P. aeruginosa and S. aureus were observed resistant against all extracts. These results are in agreement with the findings of Vishnu and Sumathi [4] and Syed et al. [5]. The ethanol extract of C. vulgaris was reported to be effective against Klebsiella sp. [5]. The variation in the susceptibility of tested pathogens against the same extract was probably due to the phylogeny of the bacterial species [11]. Generally, Gram-negative bacteria are less sensitive to antibiotics because of the complex structure of cell wall which makes the penetration of chemical difficult [12]. However, in the present study E. coli was found most sensitive to the C. vulgaris extract. Several studies reported that the extracts of C. vulgaris are much more effective against Gram-negative bacteria than gram-positive bacteria [3,5,13]. The susceptibility of Gram-negative bacteria may be due to the fatty acids present in the extract. The fatty acids are known to have antibacterial potential. Pratt et al. [14] observed that the chlorellin (a mixture of fatty acid) isolated from chlorella was effective against Gram-negative as well as Gram-positive bacteria.
The SEM micrographs of E. coli treated with diethyl ether extract clearly shows the deformation of cells with some other morphological defects such as cells have depressions and the surface of cells was rough and corrugated. Thus, it supports the observation that the diethyl ether extract of C. vulgaris is directly affecting the cell integrity of the bacteria. This may be due to a disruption of the cellular membrane of E. coli which may have led to the killing of the pathogen. Burt [15] observed similar changes in E. coli cell after treatment with essential oils. The mechanism of action for the morphological changes was expected to be the damage of the cell wall, disruption of cellular membrane or its proteins which may lead to the leakage of cell contents and coagulation of cytoplasm. The result of GC-MS analysis of the crude extract of C. vulgaris showed the presence of Methyl ester-fatty acid which is a 17 carbon atom long chain fatty acid. It has been reported that the fatty acids with carbon atom chain longer than ten can induce lysis of protoplast [3]. As mentioned earlier, chlorellin, a fatty acid isolated from C. vulgaris reported to possess antibacterial activity [14]. Besides that bio-medically important heptanal compound [16] was detected in the extract of C. vulgaris, probably this compound has played some role in the inhibition of growth of pathogens. The findings of the present study thus, suggest that diethyl ether extract of C. vulgaris could be utilized effectively as an antibacterial agent. This study will be the source for the possible discovery of new natural antibiotics for the treatment of human pathogenic bacteria. Further, investigations are required for isolation and purification of the active compound.
Acknowledgement
The Authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-086.
References
- Mendes RL, Nobre BP, Cardoso MT, Pereira AP Palabra AF. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. InorgChimActa 2003; 356: 328-334.
- Mayer AMS, Hamann MT. Marine pharmacology in 2001-2002: marine compounds with antihelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp BiochemPhyco, Part C 2005; 140: 265-286.
- Pradhan J, Das S, Das BK. Antibacterial activity of freshwater microalgae: A review. Afr J Pharm Pharmacol 2014; 8:809-818.
- Vishnu N, Sumathi R. Isolation of fresh water microalgae Chlorella sp. and its antimicrobial activity on selected pathogens. Int J Adv Res BiolSci 2014; 1: 36-43.
- Sayed S, Arasu A, Ponnusw I. The uses of Chlorella vulgaris as antimicrobial agent and as a diet: the presence of bio-active compounds which caters the vitamins, minerals in general. Int J Bio Sci Bio Tech 2015; 7: 185-190.
- Rippka R, Deruelles J, Waterburg JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteira. J Gen Microbiol 1979; 111: 1-16.
- Farnkmolle WP, Larsen, LK, Caplan FR, Patterson GML, Knubel G. Antifungal cyclic peptides from the terrestrial blue-green alga Anabaena laxa. I. isolation and biological properties. J Antibiot 1992; 45: 1451-1457.
- Chetsumon A, Fujieda K, Hirata K, Yagi K, Miura Y. Optimization of antibiotic production by the cyanobacteriumScytonema sp. TISTR 8208 immobilized on polyurethane foam. J ApplPhycol 1993; 5: 615-622.
- Herrero M, Ibañez E, Cifuentes A, Reglero G, Santoyo S. Dunaliellasalina microalga pressurized liquid extracts as potential antimicrobials. J Food Prot 2006; 69: 2471-2477.
- Bloor S, England RR. Antibiotic production by cyanobacteriumNostocmuscorum. J ApplPhycol 1989; 1: 367-372.
- Philip K, Sinniah SK, Muniandy S. Antimicrobial peptides in aqueous and ethanol extracts from microbial, plant and fermented sources. Biotechnol 2009; 1:1-16.
- Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles J. Bacteriol 1999; 181: 4725-4733.
- Demiriz T, Cokmus C, Pabuccu K. Antimicrobial activity of some algal species belonging to cyanobacteria and chlorophyta. 2011; 23: 1384-1386.
- Pratt R, Daniel TC, Eier JB, Gunnison JB, Kumler WD, Oneto JF, Strait L A, Spoehr HA, Hardin G J, Milner HW, Smith H, Strain HH. Chlorellin. An antibacterial substance from chlorella. Science 1944; 99: 351-352.
- Burt SA, Reinders RD. Antimicrobial activity of selected plant essential oils against Escherichia coli O157:H7. LettApplMicrobiol 2003; 36: 162-167.
- Cueto R, Squadrito GL, Bermudez E, Pryor WA. Identification of heptanal and nonanal in broncho alveolar lavage from rats exposed to low levels of ozone. BiochemBiophys Res Commun 1992; 188: 129-134.