Research Article - Biomedical Research (2017) Volume 28, Issue 2
Anti tumor activity of stachydrine by inhibiting histone diacetylase enzyme in gastric cancer
Ning Ma1#, Fu-Qing Wei1# and Jin-rui Guo2*1Department of General Surgery, Daqing Oil Field Hospital, Daqing, Heilongjiang 163000, China
2Department of Cardiology, Affiliated Hospital of Chende Medical College, Chende, Hebei 067000, China
#These authors contributed equally to this work
- *Corresponding Author:
- Chun-Hai Zhang
Department of Cardiology
Affiliated Hospital of Chende Medical College
PR China
Accepted on June 30, 2016
Abstract
Present investigation evaluates the anti tumor activity of stachydrine by inhibiting histone diacetylase enzyme in gastric cancer. Gastric cancer was induced by chronic administration of 1-Methyl-3-nitro-1- nitrosoguanidine (MNNG) for 40 weeks. Thereafter rats were treated with stachydrine (5 and 10 mg/kg, IP) for 8 week. Antitumor activity was assessed at the end of protocol by estimating lactate dehydrogenase (LDH) concentration in the serum and Cell cycle, apoptosis ratio, HDAC activity, expression of acetylated H3&4 proteins, markers of oxidative stress [superoxide dismutase (SOD) & glutathione peroxidase (GSH-Px)], cytokines and histopathological study of gastrointestinal tissues was also done in MNNG induced gastric cancerous rats. There were significantly (p<0.01) decreases the concentration of LDH in serum of stachydrine treated rats compared to MNNG induced gastric cancerous rats. It was observed that alteration in the cell cycle and apoptosis in the gastrointestinal tissues with the stachydrine treatment compared to cancerous rats. Moreover, activity of HDAC, markers of oxidative stress and level of cytokine significantly (p<0.01) decreases in the gastrointestinal tissues of stachydrine treated rats compared MNNG induced gastric cancerous rats. Histopathology of gastric tissue suggested that stachydrine treatment reverse the hyperplasia, metaplasia in MNNG induced gastric cancerous rat. Present study concludes that stachydrine ameliorates the gastric cancer induced MNNG in rats on the virtue of its HDAC inhibition activity.
Keywords
Stachydrine, 1-Methyl-3-nitro-1-nitrosoguanidine (MNNG), Gastric cancer, Histone diacetylase enzyme (HDAC).
Introduction
Gastric tumor is the cancer develops through epithelial cells of stomach and it is the 2nd most cause of mortality throughout the world by malignant diseases [1]. Increased level of oxidative stress and inflammatory mediators resulted in the development of cancer and the antioxidant drugs found to ameliorates the gastric cancer [2]. Histone deacetylase (HDAC) are the enzymes which control the expression of gene and functions of different proteins. HDAC inhibitor decreases the expressions of inflammatory mediators like p53, cytokines and NFκB [3]. Previous study reported that inhibition of HDAC enzyme reduces the oxidative stress and thus manages the chronic disorders [4]. It is reported that expressions of HDAC increases in all types of cancer cells including gastric cancer and thus HDAC inhibitors effectively treat the cancer [5,6]. Drug used in the management of cancer has several side effects due to which several natural products were investigated for the management of cancer. Stachydrine is an alkaloid isolated from Leonurus cardiaca L. (Lamiaceae). Previous studies reported that stachydrine possesses a strong hypoglycemic and in cardiovascular diseases [7,8]. Reports study proves stachydrine’s beneficial effect on endothelial and it also ceases the synthesis of inflammatory mediators [9]. A fluorimetric assay identified the HDAC inhibitors from a herbal extract of Leonurus cardiaca L. by enzymatic assay [10]. On the basis of this the present study investigates the anti tumor activity of stachydrine by inhibiting HDAC enzymes in gastric cancer.
Material and Methods
Animals
Sprague-Dawley rats pregnant were procured from the Avian Disease Research Center, Sichuan Agricultural University, China. Animals were kept under controlled condition as per the guidelines (cycle of 12 hr light/day, pathogen free). All the experimental procedures on animals were approved by ethical committee of the institution.
Induction of gastric cancer
Gastric cancer was induced by MNNG (Sigma, USA) at a concentration of 100 mg/L in the drinking water of rats for the period of 40 weeks. All the rats selected for the study were divided into two groups. Group I was treated with vehicle and Group II rats were treated with MNNG in drinking water for 40 week. Thereafter Group II rats were separated in to three groups. Group IIa was treated only with MNNG whereas IIb & IIc groups were treated with 5 mg/kg and 10 mg/kg of stachydrine through ip injection for the period of 8 weeks. All the rats were sacrificed at the end of the study [11]. Blood sample of all the rats were collected before the scarifying them for the estimation of lactate dehydrogenase level in the serum. Whereas, histopathological evaluation, apoptosis analysis and HDAC expression was estimated in the gastric tissue isolated from rats.
Estimation of lactate dehydrogenase
Lactate dehydrogenase activity in the rat serum was estimated by LDH kit. Activity of lactate dehydrogenase was estimated by measuring the absorbance at 340 nm using UV spectrophotometer [11].
Evaluation of status of cell cycle and apoptosis
Stomach tissues of all the rats were slices and epithelial surface of it shaved completely. There after gastric cells were separated by trisinization and neutralized by adding FBS. Thereafter cells were collected by filtering through a mesh. Collected cells were washed with phosphate buffered saline and centrifuged the cells were fixed for 1 hr in PBS. Later cells were centrifuged and incubated for 10 min time with RNase. Cells were stained by using propidium iodide for the period of 15 min and analysis was done by using flow cytometer (FACScalibur, USA) [11].
Tissue preparation for protein analysis
Gastrointestinal tissues were homogenate with ice cold buffer. The composition of buffer is 2 mM EDTA and protease inhibitor (1%) mixed in tris buffer (TBS). The fraction of nuclear protein was extracted out by centrifuging it at 800 xg for the period of 10 min and cytosolic protein by centrifuging at 9200 xg for the period of 15 min. The supernatant was used for the estimation of protein.
Estimation of HDAC activity
Colorimetric detection was used for the estimation of HDAC activity by using kit (Upstate, CA). HDAC activity expressed as % of group I by measuring the absorbance at 405 nm. Effect of stachydrine on expressions of acetylated H3 and H4 was estimated by using western blot analysis [12].
Estimation of biochemical analysis
The supernatant of tissue homogenate was used for the estimation of cytokines by specific enzyme-linked immunosorbent assay (ELISA) kits (Askenazi et al.; Ozdulger et al.). Activity of SOD & GSH-Px in the gastrointestinal tissues of MNNG induced gastric cancer in rats was estimated by spectrophotometer (Shimadzu, Japan) [12].
Histopathological study
Gastric antra of all the rats were deep into 10% formalin solution. Gastric tissues were dehydrated and then entrenched in paraffin. Section of gastric tissue was stained by with Hematoxylin and Eosin (H&E) and histopathological changes were estimated at 40X [11].
Result
Estimation of lactate dehydrogenase
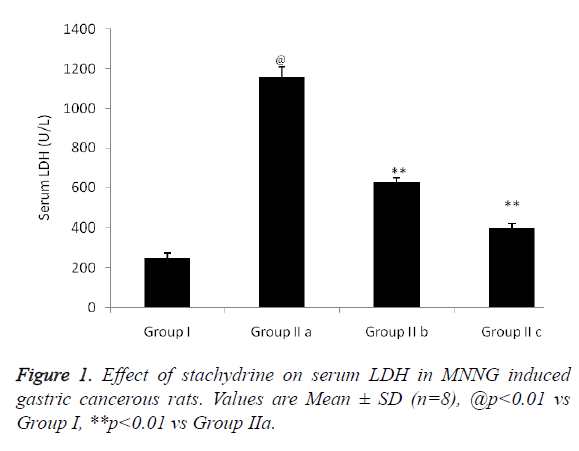
Effect of stachydrine on serum LDH in MNNG induced gastric cancerous rats was shown in Figure 1. There was significant increase (p<0.01) in the serum LHD level up to 1150 U/L compared to group I normal rats. Whereas, stachydrine treatment in group IIb and group IIc with dose of 5 mg/kg & 10 mg/kg respectively decreases increased serum LDH level compared to group IIa rats.
Estimation of cell cycle and apoptosis ration
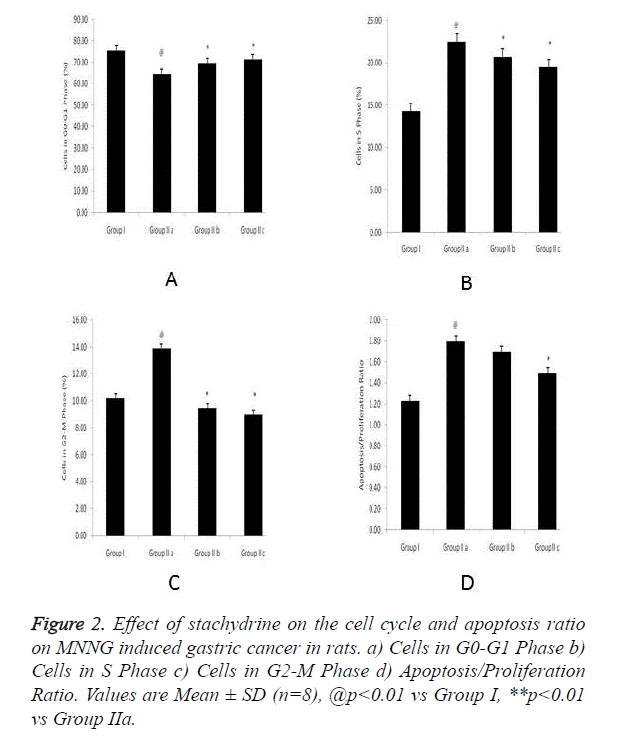
Effect of stachydrine on the cell cycle and apoptosis ratio on MNNG induced gastric cancer in rats was shown in Figure 2. There was increase on G0-G2 phase cells and increase in G2- M phase & S phase cell in group IIa rats. These alterations in cell cycle and apoptosis ration found to be altered with stachydrine treatment.
Effect of stachydrine on HDAC activity
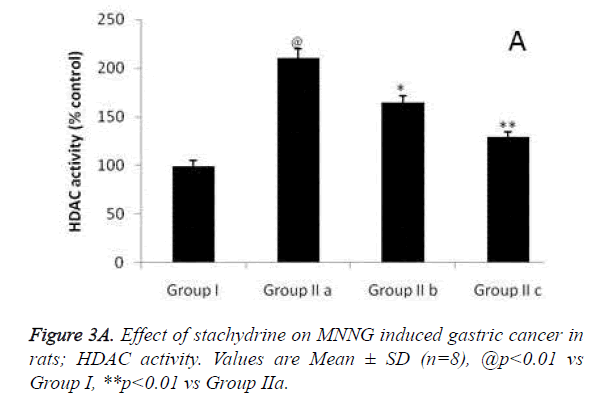
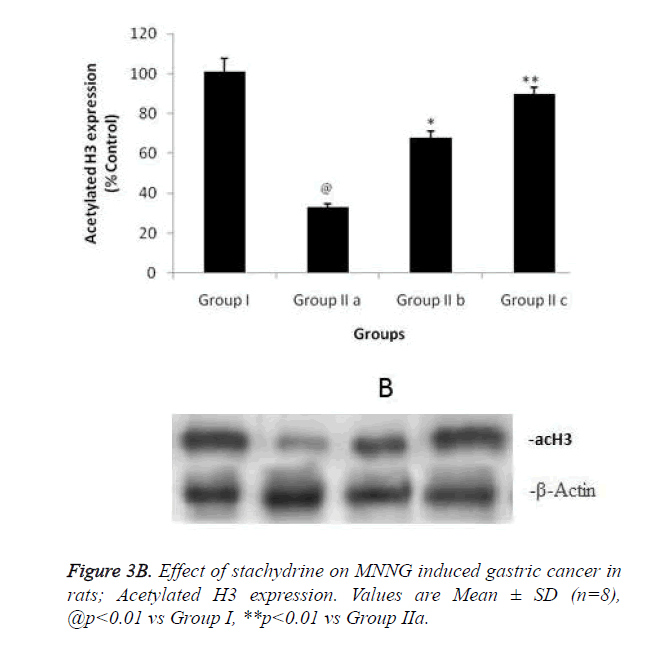
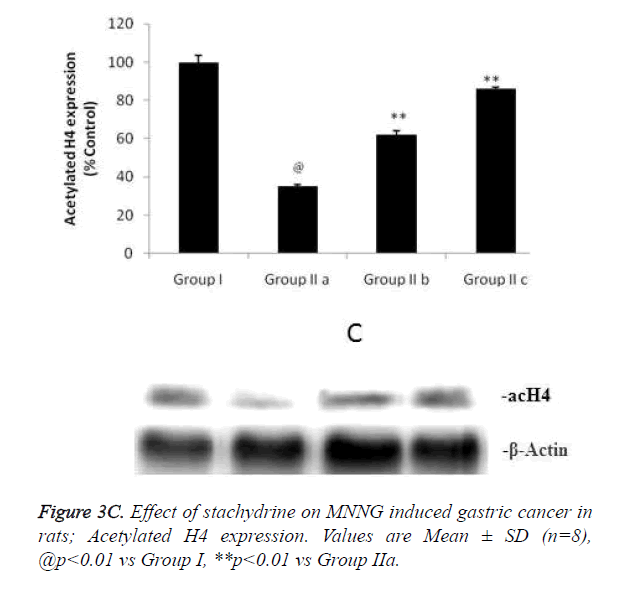
Hypothesis of our study was confirmed by estimating the effect of stachydrine on HDAC activity in the gastrointestinal tissues of MNNG induced gastric cancer in rats as shown in Figure 3a. It was observed that stachydrine decreases the activity of HDAC enzyme in dose dependent manner in the gastrointestinal tissues of MNNG induced gastric cancer in rats. Moreover the effect of stachydrine on the expression of acetylated H3 and H4 protein in the gastrointestinal tissues of MNNG induced gastric cancer in rats were determines in this study (Figures 3b and 3c). There were significant (p<0.01) increases in the expression of acetylated H3 and 4 proteins with the stachydrine treatment in the GIT cancerous tissues of rats compared to group IIa.
Estimation of Cytokines in the gastrointestinal tissues
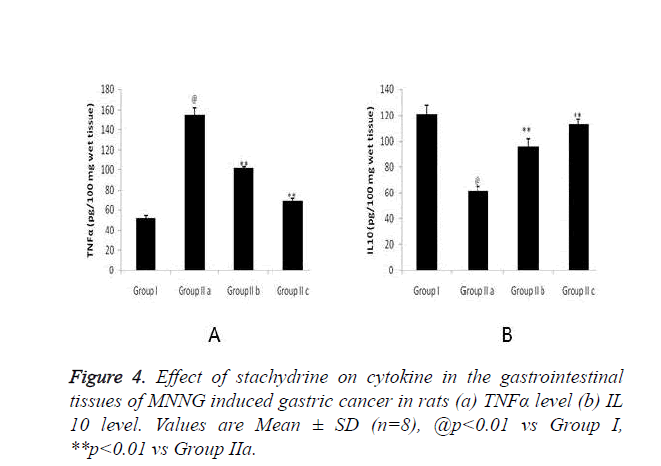
Figure 4 shows effect of stachydrine on the cytokine level in gastrointestinal tissues of MNNG induced gastric cancer in rats. There were significant (p<0.01) increase in the level of TNFα in the brain tissues which confirms the cancer in MNNG induced gastric cancer in rats compared to group I. Whereas, stachydrine ameliorates the cancer of gastrointestinal tract by significant (p<0.01) reduction in the level of TNFα compared to group IIa rats (Figure 4a). Moreover the level of IL 10 found to be increased significantly (p<0.01) in stachydrine treated group of rats compared to group IIa (Figure 4a). Effect of satchydrine on the TNFα and IL 10 were found to be in a dose dependent manner.
Estimation of oxidative stress in gastrointestinal tissues
Markers of oxidative stress parameters such as SOD and GSH in the gastrointestinal tissues of MNNG induced gastric cancer in rats were found to be decreased compared to group I rats. It was observed that treatment with the stachydrine significantly increases (p<0.01) in the SOD and GSH-PX level in the gastrointestinal tissues and thereby it decreases the oxidative stress in MNNG induced gastric cancer in rats as shown in Table 1.
| Sr. No. | Group | SOD (U/100 mg wet tissue) | GSH-PX (U/100 mg wet tissue) |
|---|---|---|---|
| 1 | Group I | 2.67 ± 0.23 | 192.7 ± 8.3 |
| 2 | Group IIa | 0.79 ± 0.12@ | 96.8 ± 5.9@ |
| 3 | Group IIb | 1.73 ± 0.19** | 130.6 ± 7.2** |
| 4 | Group IIc | 2.18 ± 0.20** | 169.2 ± 8.1** |
| Values are Mean ± SD (n=8), @p<0.01 vs Group I, **p<0.01 vs Group Iia | |||
Table 1. Effect of stachydrine on parameters of oxidative stress in the gastrointestinal tissues of MNNG induced gastric cancer in rats.
Histopathological study
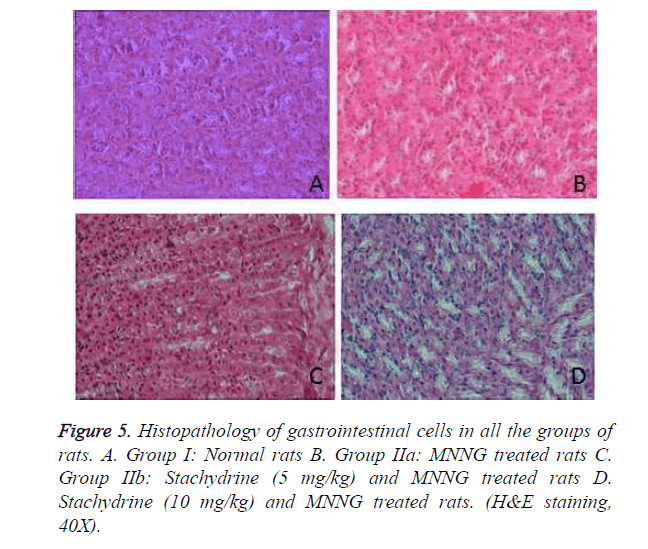
Results of histopathological studies of gastrointestinal cells in all the groups of rats were shown in Figure 5. It was observed hyperplasia, metaplasia, dysplasia and adenoma were present in the tissue section of group IIa rats. Whereas, treatment with stachydrin reduces the number of hyperplasia and metaplasia cell in MNNG treated rats and no evidences of adenoma and dysplasia found in the gastrointestinal tissues of stachydrin treated MNNG treated rats.
Discussion
Present investigation evaluates the anticancer activity of stachydrine in MNNG induced gastric cancer by inhibiting the HDAC activity in the gastrointestinal tissues. Its effect on expression of acetylated H3 and 4 proteins, markers of oxidative stress [superoxide dismutase (SOD) & glutathione peroxidase (GSH-Px)], cytokines was estimated inMNNG induced gastric cancerous rats. Globally, cancer of gastrointestinal is one of the major causes of death. Treatment options available for the management of gastric cancer are chemotherapy after surgery [13]. Limitations of chemotherapy emerge in to the development of alternative medicine for the management of gastric cancer. Acetylation of histone has a proved role in the development of cancer. Literature suggested that the expression of HDAC found to be increases in the cancerous tissue and the drug inhibits the HDAC used in the treatment of gastric cancer [14]. MNNG is an alkylating agent used for the induction of gastric cancer in animals. It is known have higher rate of cancer induction compared to other carcinogenic agents. Literature suggested that apoptosis ratio was significantly increased in MNNG induced cancerous tissues and treatment with stachydrine ameliorates the altered cell cycle and apoptosis ratio in MNNG induced gastric cancerous rats [15].
Literature reveals that increased oxidative stress and free radical generation resulted in cancer [16]. This increased free radical alters the cytokines expression and resulted in the development of cancer [17]. Drugs having antioxidant properties play a role in the prevention and treatment of cancer [18]. However, stachydrine treatment decreases (p<0.01) oxidative stress and alters the level of cytokine gastrointestinal cancerous tissues. It also decreases the HDAC activity and improves the expression of H3&4 proteins in the gastrointestinal tissues of MNNG treated rats. Decrease in the HDAC activity and increase in the expression of H3&4 proteins in gastrointestinal tissues by stachydrine treatment confirm the possible mechanism of its anti-cancer activity in gastrointestinal cancer. On the basis of all of the above property stachydrine attenuate the gastric cancer in MNNG induced gastric cancerous rats.
Conclusion
Present study concludes the anti tumor activity of stachydrine in MNNG induced gastric cancer in rats by virtue of its HDAC inhibition activity.
References
- Macdonald JS. Gastric cancer-new Therapeutic toptions. N Eng J Med 2006; 355: 76-77.
- John C, Layke PPL. Gastric cancer: diagnosis and treatment options. Am Fam Physician 2004; 69: 1133-1140.
- Halili MA, Andrews MR, Sweet MJ, Fairlie DP. Histone deacetylase inhibitors in inflammatory disease. Curr Top Med Chem 2009; 9: 309-319.
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Moan NL, Grueter CA. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013; 339: 211-214.
- Ropero S, Esteller M. The role of histone deacetylases (hdacs) in human cancer. Mol Oncol 2007; 1: 19-25.
- Bolden JE, Shi W, Jankowski K, Kan CY, Cluse L, Martin BP, MacKenzie KL, Smyth GK, Johnstone RW. HDAC inhibitors induce tumor-cell-selective pro-apoptotic transcriptional responses. Cell Death Dis 2013; 4: e519.
- Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr 2003; 78: 559S-569S.
- Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009; 169: 659-669.
- Hu YY, He KW, Guo RL. Six alkaloids inhibit secretion of IL‐1a, TXB(2), ET‐1, and E‐selectin in LPS‐induced endothelial cells. Immunol Invest 2012; 41: 261- 274.
- Krasteva S, Heiss E, Krenn L. Optimization and application of a fluorimetric assay for the identification of histone deacetylase inhibitors from plant origin. Pharma Biol 2011; 49: 658-668.
- Bathaie SZ, Miri H, Mohagheghi MA, Dizaji MM, Shahbazfar AA, Hasanzadeh H. Saffron Aqueous Extract Inhibits the Chemically-induced Gastric Cancer Progression in the Wistar Albino Rat. Iran J Basic Med Sci 2013; 16: 27-38.
- Cetinkaya M, Cansev M, Kafa IM, Tayman C, Cekmez F, Canpolat FE, Tunc T, Saric SU. Cytidine 5'-diphosphocholine (CDP-choline) ameliorates hyperoxic lung injury in a neonatal rat model. Pediatr Res 2013; 74: 26-33.
- Lynch HT, Grady W, Suriano G, Huntsman D. Gastric cancer: new genetic developments. J Surg Oncol 2005; 90: 114-133.
- Colarossi L, Memeo L, Colarossi C, Aiello E, Iuppa A, Espina V, Liotta L, Mueller C. Inhibition of histone deacetylase 4 increases cytotoxicity of docetaxel in gastric cancer cells. Proteomics Clin Appl 2014; 8: 924-931.
- Kok SH, Cheng SJ, Hong CY, Lee JJ, Lin SK, Kuo YS, Chiang CP, Kuo MYP. Norcantharidin-induced apoptosis in oral cancer cells is associated with an increase of proapoptotic to antiapoptotic protein ratio. Cancer Lett 2005; 217: 43-52.
- Tu H, Sun H, Lin Y, Ding J, Nan K, Li Z, Shen Q, Wei Y. Oxidative stress upregulates PDCD4 expression in patients with gastric cancer via miR-21. Curr Pharm Des 2014; 20: 1917-1923.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med 2010; 49: 1603-1616.
- Bennett LL, Rojas S, Seefeldt T. Role of Antioxidants in the Prevention of Cancer. J Exp Clin Med 2012; 4: 215e222.