- Biomedical Research (2010) Volume 21, Issue 3
Angiogenic factors in the pathogenesis and pathophysiology of preeclampsia: A Mini review
Suseela Yelumalai3, Kaviarasan Subramanian2, Siti Zawiah Omar3, Rajes Qvist2 and Sekaran Muniandy1*
1Department of Molecular Medicine, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia.
2Department of Medicine, University of Malaya Medical Center, University of Malaya, 50603 Kuala Lumpur, Malaysia.
3Department of Obstretics & Gynaecology, University of Malaya Medical Center, University of Malaya, 50603 Kuala Lumpur, Malaysia.
- *Corresponding Author:
- Sekaran Muniandy
Department of Molecular Medicine
Faculty of Medicine
University of Malaya
Kuala Lumpur
Malaysia-50603
Email: sekaran@um.edu.my
Accepted Date: March 11 2010
Abstract
Preeclampsia (PE) the de novo occurrence of hypertension and proteinuria after the 20th week of gestation is a major cause of maternal and fetal morbidity worldwide. While the etiology of PE is still unclear, clinical phenotypes have been associated with high circulating levels of anti-angiogenic proteins such as soluble Fms-like tyrosine kinase 1 (sFlt1) and soluble endoglin (sEng). Furthermore PE is associated with low serum free placental growth factor (PIGF) and free vascular endothelial growth factor (VEGF). Since alterations in levels of these factors precede the onset of clinical disease, have these factors may be useful to screen or identify patients at risk for PE. Women with a history of PE have an increased risk of hypertension, and cardiovascular and renal disease Therefore, this raises the possibility of measuring circulating angiogenic proteins in the blood and the urine as a diagnostic and screening tool for PE. The availability of a test to predict PE would be a powerful tool in preventing PE-induced mortality, especially in developing nations, where high-risk specialists are limited. This review will summarize our current understanding of the role of circulating angiogenic proteins in the pathogenesis and clinical diagnosis/prediction of PE.
Keywords
Preeclampsia, angiogenic factors, hypertension, pathophysiology
Introduction
Preeclampsia (PE) is a pregnancy induced hypertensive disorder that by usually affects 3% - 5% of first pregnancy and is a leading cause of maternal and fetal mortality. As many as 8,370,000 cases of PE per year are seen [1]. This disorder characterized by the development of a maternal syndrome that includes, proteinuria, haemolysis, liver abnormalities, thrombocytopenia (HELLP Syndrome), seizures (eclampsia) coagulations abnormalities, edema, and vascular abnormalities [2,3]. The underlying mechanism that places women with a history of PE at risk for hypertension and CVD remains speculative. In addition the etiology of PE remains largely unknown but it is known to be related to endothelial dysfunction. However, investigations on this disorder offer potential treatment [4].
Risk Factors
Interruption of nutrient and oxygen exchange between mother and fetus by reduced placental perfusion alone doesn’t contribute to PE [5]. Therefore, decreased placental perfusion is not the primary root cause of PE [6]. Certain maternal factors genetically, medically or environmentally, may be present to increase the susceptibility of reduced placental perfusion have increasing the risk of the development of PE or other hypertensive disorders during pregnancy [7].
Genetic Factor
The genetic cause of PE is still unclear, and genes contributing linked to PE have yet to be elucidated. However, studies have reported an association between PE and polymorphisms of genes that control hypertension, coagulations or oxidative stress metabolism, such as rennin, angiotensinogen, endothelial nitric oxide synthase (eNOS), Factor V LEIDEN, methyltetrahydrofolate or lipoprotein lipase [8-12]. A number of these factors are also associated with recurrent fetal loss [13,14] and emphasizes the contribution of PE to fetal loss. Thrombophilic mutations of the two vital thrombophilia markers, factor V Leiden (FVL) and prothrombin G20210A as a genetic factor may contribute to PE which tends to progress into recurrent pregnancy loss among the Asian population [15]. Most PE cases are seldom categorized as hereditary disease. However first degree relative of a women with PE are often at risk of this hypertensive disorder depending on the severity of the disease [16].
Maternal factors
PE is categorized a disorder of the first pregnancy. However, investigations showed that multiparous women frequently lost their protective factors from encountering PE if their pregnancy is with a new partner and said to be at risk similar to that of primiparous women [17]. The risk of PE decreases as the women is continuously exposed to specific antigens from the same partner. Although the potential risk of PE on multiparity is reduced, interpregnancy interval may lead to increased risk of encountering this disease [18]. Pre-existing maternal factors such as previous history of preeclampsia, multiple pregnancy, and nulliparity were found to increase the risk of PE.
Other Factors
Insulin resistance, obesity and thrombophilia are the other factors that influence PE [19-21]. Protein-calorie under nutrition [22] is another important risk factor which has been discovered in developing countries, and calcium supplementation does not promote/initiate the increased risk of preeclampsia [23]. Multifestal gestations and hydatidiform moles are also associated with increased preeclampsia risk [24].
Pathogenesis and Pathophysiology of PE
Role of Placenta
Vascularization is an important process which involves vasculogenesis (formation of new blood vessel) and angiogenesis (formation of growth blood vessel) for a proper formation and complete development of placenta [25]. Defects in placental perfusion is an initial event in PE leads to vascular endothelial dysfunction by the mechanisms that remain unclear [26]. It has been suggested that release of factors from the placenta in response to ischemia results in dysfunction of the endothelium of maternal circulation [27]. Studies using serum from preeclamptic women have shown that cultured endothelial cells respond with an increased expression and release of growth factors and fibronectin, induction of oxygen radicals, as well as an inhibition of prostacyclin production [28,29].
Abnormal Placentation
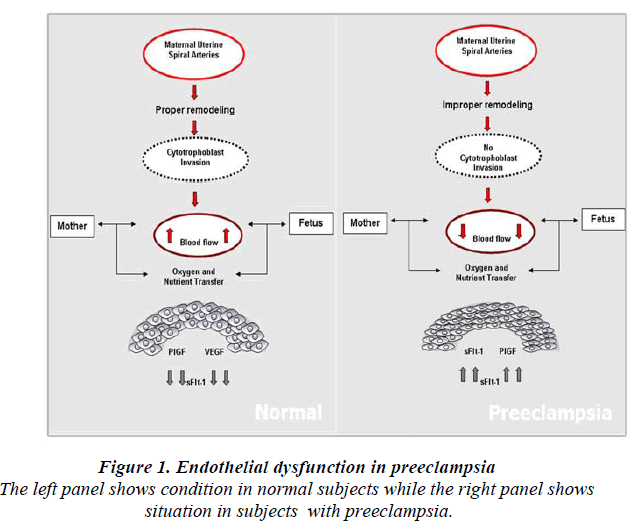
Normal placental development involves extravillous cytotrophoblastic invasion of the uterine spiral arteries of the deciduas and myometrium. The invasive fetal cells replace the endothelial layer of uterine vessels, transforming them from small resistant vessels to flaccid high caliber capacitance vessels (Fig.1). In PE, the increase in uterine blood flow needed to sustain the fetus through pregnancy is insufficient. Cytotrophoblast invasion of the arteries is limited to the superficial deciduas and the myometrial segments remain narrow [30]. Fisher et al shown that in normal placental development, invasive cytotrophoblasts downregulate the expression of adhesion molecules characteristics of their epithelial cell origin and adopt endothelial cell-surface adhesion phenotype, a process dubbed pseudovasculogenesis [31] or vascular mimicry. In PE, cytotrophoblast do not undergo this switching of cellsurface intergrins and adhesion molecules, and they fail to adequately invade the myometrial spiral arteries.
Endothelial dysfunction
Endothelial dysfunction, a central component of the pathophysiology of PE, is known to contribute in the pathogenesis of hypertension and cardiovascular diseases. Hypertension associated with PE develops during pregnancy and remits after delivery, implicating the placenta as the central cause of the disease. Intact endothelial cells have antiadhesive and anticoagulant properties, regulate vascular permeability and modulate the effect of vasoconstrictor agonist on the vessel wall [32]. Several lines of evidence support the hypothesis that dysfunction of the vascular endothelium is important in the pathogenesis of PE [33]. The vascular response to vascontrictors, the tendency for coagulation [34], permeability of capillaries [35] and the plasma concentration of fibronectin and endothelin is greatly enhanced in preeclamptic women [36,37]. Although some studies have reported no significant changes in circulating levels of endothelin during pregnancy-induced hypertension, a role for endothelin as a paracrine or autocrine agent in PE remains worthy of consideration.
Angiogenic Factors
Soluble Fms-like tyrosine Kinase-1, VEGF and PIGF
Hypertension and proteinurea the hallmarks of PE, occur due to excess circulating anti-angiogenic peptides produced by the placenta, which is the soluble fms-like tyrosine kinase 1 (sFlt-1, also referred to as sVEGFR-1) (antiangiogenic factors) [25]. In context to pro-angiogenic proteins such as vascular endothelial growth factor (VEGF) and placental growth factor (PIGF), the presence of sFlt-1 has been shown to cause hypertension, proteinurea and glomerular endotheliosis in rats [37]. Studies have shown that patient diagnosed with PE had an elevated level of sFlt-1 in maternal serum or plasma compared to the pro-angiogenic factors [25].
sFlt-1 a tyrosine kinase protein which disables blood vessel growth [38] and is an alternatively spliced variant of vascular endothelial growth factor receptor 1 (VEGF-R1).
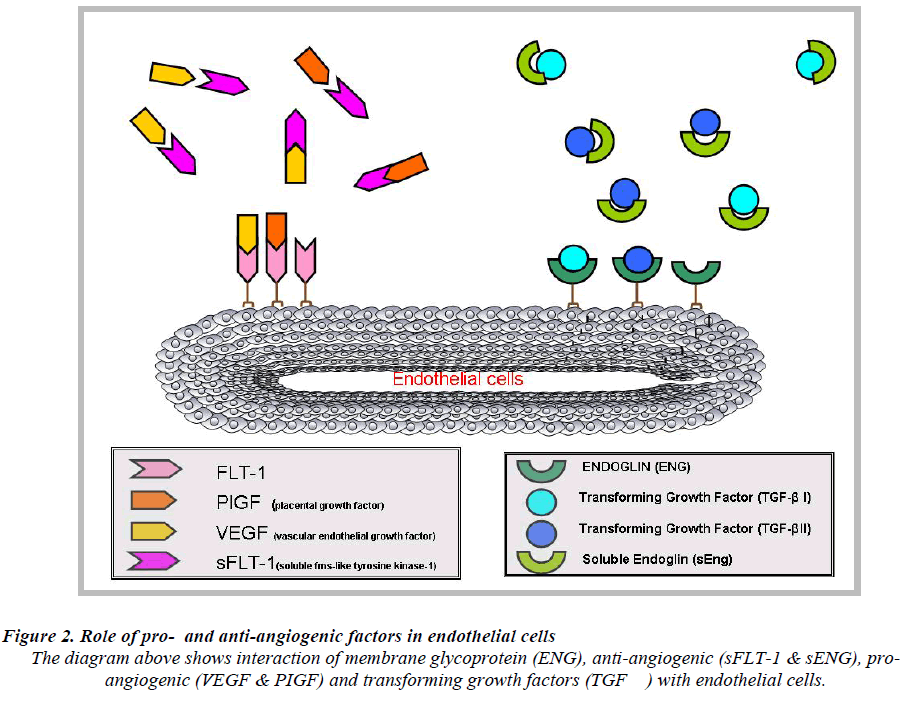
It acts as a VEGF and PIGF antagonist [39] by preventing the interaction of the pro-angiogenic factors with the endothelial receptors on the cell surface and induces endothelial dysfunction which subsequently leads to PE (Fig.2). Elevated levels of circulating angiogenic proteins/ factors in the maternal serum or plasma in pregnancy would discriminate normal pregnancy from PE. Levels of sFlt-1 remain stable during gestational age of 16-20 weeks and increase steadily during the 24-28 weeks of gestation period until term [40]. In PE, the levels of sFlt-1 are found to be highly increased as compared to PIGF levels [25]. These data suggest that, sFlt-1 to PIGF ratio could be a predictive marker for the early onset of PE rather than measuring sFlt-1 or PIGF levels alone [41].
Endoglin and Transforming Growth Factors
Endoglin (ENG) also known as CD105 [42], was earlier discovered by a monoclonal antibody (44G4) raised against a pre-B lymphobalstic HOON cell line [43]. ENG encodes for type 1 integral membrane glycoprotein [44] and mutations in ENG cause an autosomal-dominant disorder disease known as hereditary haemorrhagic telangiectasia type 1 (HHT1), this disorder is characterized by arteriovenous malformations and focal loss of capillaries [45]. ENG functions as an receptor for several transforming growth factor super family members [46]. ENG act as a protein modulator of TGF-β signaling by interaction with TGF-β1 and TGF-β2 [47]. It is identified as an accessory receptor for TGF-β as it plays a versatile role in tumor angiogenesis [45,48]. In PE [42], focus on ENG is due to the fact that ENG has a major contribution to vasculogenesis and disease [49].
TGF-β a family of multifunctional proteins, includes three TGF-β isoforms (TGF-β1, TGF-β2 and TGF-β3), activins and bone morphogenetic proteins (BMP’s) which are involved in many different pathophysiological processes including development, wound healing, cancer, fibrosis, vascular, and immune disease [49,50]. The signaling pathway plays a very important role in vascular morphogenesis as the investigation using targeted inactivation has shown. Signaling occurs via two transmembrane type I and type II receptors endowed with serine/threonine kinase activity [51,52]. TGF-β interacts with TGF- β type II receptor (TβRII) and TGF-β type I receptor (TβRI) also know as activin receptor-like kinase 5 (ALK-5) [53].
As an auxiliary receptor, [54] it act as signaling response modulator protein of multiple members of the TGF-β family [55] and involved in TGF-β independent signaling [56]. Futhermore, a soluble form of endoglin (sEng) has been found, most likely generated by proteolytic shedding, which antagonizes the membrane bound form [42]. Taken together, multiple layers of complexity exist by which the function of endoglin is regulated.
Soluble Endoglin (sEng)
Placental endoglin(ENG), a member of TGF-β family is found overexpressed in PE and circulates as soluble endoglin (sEng) in maternal sera/plasma [39,57]. Circulatory levels are dependent on the severity of PE whereby concentration of sEng is much higher as in PE compared to normal cases [25,42].sEng an anti-angiogenic factor, acts as a TGF-β antagonist where it adheres to the TGF- β1 and TGF-β3 receptor and blocks these molecules from binding to the endothelial cell surface, [58] and consequently suppresses the pro-angiogenic and vasodilatory effects of TGF-β1 / TGF-β3 leading to endothelial dysfunction [46].
Soluble endoglin (sEng) acts as an antagonist to TGF-β families by preventing endothelial capillary tube formation and promotes vascular permeability [42]. Overexpression of sEng by adenoviral vector in rats is associated with milder proteinuria and hypertension as compared to overexpression of sFlt-1 alone [25]. Co-expression of sEng and sFlt-1 in rats results in the development of severe proteinuria, hypertension, intrauterine growth restriction, lower platelet counts and elevated LDH similar to that in the HELP syndrome [42].These data suggest that sEng and sFlt-1 both causes endothelial dysfunction by different mechanism but may act in concert to produce to the clinical syndrome of PE [25].
Altered levels of circulating angiogenic factors, especially sFlt1, sEng, and PlGF, have been reported to precede the onset of preeclampsia. Excessive levels of circulating anti-endothelial factors produced by the abnormal placenta cause generalized endothelial dysfunction prominent in the maternal syndrome, but the origins of abnormal placentation and its specific role in preeclampsia are still not well understood. Prospective studies to characterize the role of circulating proteins with mediators of endothelial dysfunction, such as sFlt-1, should help shed light on the pathologic mechanisms of the maternal syndrome.
Acknowledgement
Authors are grateful to Ministry of Science, Technology and Innovation (MOSTI), Malaysia for supporting this work.
References
- Lenfant C. Working group report on high blood pressure in pregnancy. J Clin Hypertens (Greenwich). 2001; 3: 75-88.
- Eskenazi B, Fenster L, Sidney S, Elkin EP. Fetal growth retardation in infants of multiparous and nulliparous women with preeclampsia. Am J Obstet Gynecol. 1993; 169: 1112-1118.
- Stone JL, Lockwood CJ, Berkowitz GS, Alvarez M, Lapinski R, Berkowitz RL. Risk factors for severe preeclampsia. Obstet Gynecol. 1994; 83: 357-361.
- Roberts JM, Pearson GD, Cutler JA, Lindheimer MD. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertens Pregnancy. 2003; 22: 109-127.
- Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005; 46: 1243-1249.
- Alexander BT. Prenatal influences and endothelial dysfunction: a link between reduced placental perfusion and preeclampsia. Hypertension. 2007; 49: 775-776.
- Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008; 59: 61-78.
- Ward K, Hata A, Jeunemaitre X, Helin C, Nelson L, Namikawa C, et al. A molecular variant of angiotensinogen associated with preeclampsia. Nat Genet. 1993; 4: 59-61.
- Arngrimsson R, Hayward C, Nadaud S, Baldursdottir A, Walker JJ, Liston WA, et al. Evidence for a familial pregnancy-induced hypertension locus in the eNOSgene region. Am J Hum Genet. 1997; 61: 354-362.
- Dizon-Townson DS, Nelson LM, Easton K, Ward K. The factor V Leiden mutation may predispose women to severe preeclampsia. Am J Obstet Gynecol. 1996; 175: 902-905.
- Sohda S, Arinami T, Hamada H, Yamada N, Hamaguchi H, Kubo T. Methylenetetrahydrofolate reductase polymorphism and pre-eclampsia. J Med Genet. 1997; 34: 525-526.
- Omar SZ, Qvist R, Khaing SL, Muniandy S, Bhalla S. Thrombophilic mutations in pre-eclampsia and pregnancy- induced hypertension. J Obstet Gynaecol Res. 2008; 34: 174-178.
- Ayadurai T, Muniandy S. Thrombhophilia abnormalities in recurrent pregnancy loss. Biomedical Research. 2010; 21 (1): 87-89.
- Ayadurai, T, Ayob Y, Muniandy S, Omar SZ. Inherited thrombophilia markers in Malaysian women with recurrent fetal loss. Thrombosis Hemostasis 2007; 98: 1152-1154.
- Ayadurai T, Muniandy S, Omar SZ. Thrombophilia investigation in Malaysian women with recurrent pregnancy loss. J Obstet Gynaecol Res. 2009; 35 (6): 1061- 1068.
- Carr DB, Epplein M, Johnson CO, Easterling TR, Critchlow CW. A sister's risk: family history as a predictor of preeclampsia. Am J Obstet Gynecol. 2005; 193: 965-972.
- Tuffnell DJ, Jankowicz D, Lindow SW, Lyons G, Mason GC, Russell IF, et al. Outcomes of severe preeclampsia/ eclampsia in Yorkshire 1999/2003. BJOG. 2005; 112: 875-880.
- Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002; 346: 33-38.
- Walker JJ. Pre-eclampsia. Lancet. 2000; 356:1260-5.
- Wolf M, Sandler L, Munoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: a prospective study. J Clin Endocrinol Metab. 2002; 87: 1563-1568.
- Kupferminc MJ, Eldor A, Steinman N, Many A, Bar- Am A, Jaffa A, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med. 1999; 340: 9-13.
- Brewer T. Letter: Role of malnutrition in pre-eclampsia and eclampsia. Am J Obstet Gynecol. 1976; 125: 281- 282.
- Lopez-Jaramillo P, Casas JP, Serrano N. Preeclampsia: from epidemiological observations to molecular mechanisms. Braz J Med Biol Res. 2001; 34: 1227- 1235.
- Slim R, Mehio A. The genetics of hydatidiform moles: new lights on an ancient disease. Clin Genet. 2007; 71: 25-34.
- Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008; 75: 1-8.
- Friedman SA, de Groot CJ, Taylor RN, Golditch BD, Roberts JM. Plasma cellular fibronectin as a measure of endothelial involvement in preeclampsia and intrauterine growth retardation. Am J Obstet Gynecol. 1994; 170: 838-841.
- DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999; 155: 293-301.
- Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989; 161: 1200-1204.
- Baker PN, Davidge ST, Barankiewicz J, Roberts JM. Plasma of preeclamptic women stimulates and then inhibits endothelial prostacyclin. Hypertension. 1996; 27: 56-61.
- Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe preeclamptic pregnancies. Br J Obstet Gynaecol. 1994; 101: 669-674.
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997; 99: 2139-2151.
- Thadhani R, Stampfer MJ, Hunter DJ, Manson JE, Solomon CG, Curhan GC. High body mass index and hypercholesterolemia: risk of hypertensive disorders of pregnancy. Obstet Gynecol. 1999; 94: 543-550.
- Said J, Dekker G. Pre-eclampsia and thrombophilia. Best Pract Res Clin Obstet Gynaecol. 2003; 17: 441- 458.
- Xiong X, Wang FL, Davidge ST, Demianczuk NN, Mayes DC, Olson DM, et al. Maternal smoking and preeclampsia. J Reprod Med. 2000; 45: 727-732.
- Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia - a state of sympathetic overactivity. N Engl J Med. 1996; 335: 1480-1485.
- Chesley LC, Talledo E, Bohler CS, Zuspan FP. Vascular Reactivity to Angiotensin Ii and Norepinephrine in Pregnant Women. Am J Obstet Gynecol. 1965; 91: 837- 842.
- Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973; 52: 2682-2689.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111: 649-658.
- De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D'Anna R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand. 2008; 87: 837-842.
- Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005; 46: 1077-1085.
- Buhimschi CS, Norwitz ER, Funai E, Richman S, Guller S, Lockwood CJ, et al. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am J Obstet Gynecol. 2005; 192: 734-741.
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006; 12: 642- 649.
- Quackenbush EJ, Gougos A, Baumal R, Letarte M. Differential localization within human kidney of five membrane proteins expressed on acute lymphoblastic leukemia cells. J Immunol. 1986; 136: 118-124.
- Gougos A, Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. 1990; 265: 8361-8364.
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994; 8: 345-351.
- Lopez-Novoa JM. Soluble endoglin is an accurate predictor and a pathogenic molecule in pre-eclampsia. Nephrol Dial Transplant. 2007; 22: 712-714.
- Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factorbeta receptors I and II. J Biol Chem. 2002; 277: 29197- 29209.
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999; 284: 1534-1537.
- ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007; 8: 857-869.
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000; 342: 1350-1358.
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997; 390: 465-471.
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007; 8: 970-982.
- Franzen P, ten Dijke P, Ichijo H, Yamashita H, Schulz P, Heldin CH, et al. Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. Cell. 1993; 75: 681-692.
- Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992; 267: 19027- 19030.
- Lastres P, Letamendia A, Zhang H, Rius C, Almendro N, Raab U, et al. Endoglin modulates cellular responses to TGF-beta 1. J Cell Biol. 1996; 133: 1109-1121.
- Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGFbeta receptor function in the endothelium. Cardiovasc Res. 2005; 65: 599-608.
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006; 355: 992-1005.
- Luft FC. Soluble endoglin (sEng) joins the soluble fmslike tyrosine kinase (sFlt) receptor as a pre-eclampsia molecule. Nephrol Dial Transplant. 2006; 21: 3052- 3054.