Research Article - Biomedical Research (2017) Volume 28, Issue 21
Analysis on risk factors for cerebral hyperperfusion syndrome following carotid angioplasty stenting in the elderly patients with carotid artery stenosis and intervention measures
Rui Wang1, Shuangchun Sun1 and Xinxin Fan2*
1Xi'an Central Hospital, Shaanxi, China
2Xi’an No.3 Hospital Xi’an, Shaanxi, China
Accepted date: December 28, 2017
Abstract
Objective: To study the risk factors for Cerebral Hyperperfusion Syndrome (CHS) following Carotid Angioplasty Stenting (CAS) in the elderly patients with carotid artery stenosis and to explore the intervention measures for prevention and treatment of CHS.
Methods: A total of 500 elderly patients with carotid artery stenosis enrolled in our hospital from May 2012 to May 2017 were selected as the objects. They all were treated with CAS, and divided according to presence of CHS into non CHS group (n=482) and CHS group (n=18), general data of patients were compared between the two groups, and independent risk factors were analysed by stratified method.
Results: The Univariate analysis showed that there was statistically significant difference between CHS group and non CHS group in diabetes and postoperative hypertension (P<0.05); but not in age, sex, hypertension, hyperlipidemia, ischemic events, drinking history as well as degree of stenosis (P>0.05). CAS was performed on 532 cases in the two groups, including 18 cases in CHS group and 514 cases in non CHS group. There was no statistically significant difference between the two groups in operative MCA occlusion, operative PCoA opening, symptomatic focus, and operative ACAA1 segment dysgenesis (P>0.05). The stratified analysis showed that postoperative hypertension (OR=7.248, P<0.01) and diabetes mellitus (OR=5.906, P<0.01) were important risk factors for CHS after CAS.
Conclusion: In elderly patients with carotid artery stenosis, those with postoperative hypertension or diabetes history are more prone to CHS following CAS with poor prognosis, which should be controlled and prevented from a variety of perspectives through intervention measures.
Keywords
Elderly, Carotid artery stenosis, Carotid angioplasty stenting, Cerebral hyperperfusion syndrome, Risk factors, Hypertension, Diabetes mellitus
Introduction
Carotid stenosis is the stenosis of carotid artery that serves as the main blood vessel from the heart to the brain and other parts of the head. Carotid stenosis is mostly caused by atherosclerotic plaques of the carotid artery, with a high incidence and often occurs in the common carotid bifurcation and the beginning of the internal carotid artery. The main causes of carotid stenosis are atherosclerosis, aortic inflammation and fibromuscular dysplasia; other causes include trauma, arterial torsion, congenital atresia, tumor, artery or periarteritis, and fibrosis after radiotherapy. Carotid artery stenosis is an important cause of ischemic stroke, accounting for 5% to 10%. It is generally treated with Carotid Angioplasty Stenting (CAS), Carotid Endarterectomy (CEA) and internal medicine [1]. Some clinical trials and many retrospective analysis by medical centers show that [2] CAS and CEA both were the main methods for treatment of carotid stenosis in the past decades with better curative effect than medicine therapy alone. With the development of endovascular interventional treatment in recent years, the safety and effectiveness of CAS have been confirmed by some large-scale randomized controlled trials [3]. However, some patients treated with CAS are prone to such complications as ischemic stroke, immediate hemodynamic changes, acute or subacute thrombosis and Cerebra1 Hyperperfusion Syndrome (CHS) [4,5]. In particular, CHS, although with low incidence rate, has rapid progress of the condition, which is very easy to induce intracranial hemorrhage and death in patients [6]. Therefore, a comprehensive treatment program should be developed for those with high risk of CHS before CAS is performed on patients with carotid stenosis to prevent and control the occurrence or development of CHS so as to ensure the safety of high-risk patients. This study retrospectively analysed the general data of 500 elderly patients with carotid artery stenosis treated by CAS in our hospital from May 2012 to May 2017, aiming to find out the main risk factors of CHS after CAS, with the details reported as follows.
Subjects and Methods
Research subjects
A total of 500 elderly patients with carotid artery stenosis enrolled in our hospital from May 2012 to May 2017 were selected as the objects. Among them there were 430 males and 70 females aged 61-79 with an average age of (68.3 ± 3.5 y), including 460 cases of unilateral CAS and 40 cases of bilateral CAS. All patients were diagnosed by color Doppler ultrasound. Inclusion criteria: (1) the degree of residual stenosis after CAS (calculated by NASCET method) was below 30 %; (2) CAS stents were all self-expandable; (3) stent was located in sinus of internal carotid. Exclusive criteria: (1) patients with antiplatelet drug allergy, bleeding tendency or accompanied by malignant tumor or organ dysfunction; (2) patients with incomplete cerebral angiography data and case information; (3) patients simultaneously with posterior circulation stent. The enrolled 500 patients were divided into non CHS group (n=482) and CHS group (n=18) according to presence of CHS.
Research methods
A retrospective analysis was conducted on such baseline data as patient gender, age, history of heart disease, diabetes, hypertension, hyperlipidemia, smoking history, drinking history, history of stroke, perioperative hemodynamic depression, presence of bilateral CAS and presence of Transient Ischemic Attack (TIA) followed by a record of such characteristics as degree of stenosis in the operative side, presence of operative Middle Cerebral Artery (MCA) occlusion, Operative Posterior Communicating Artery (PCoA) open, asymptomatic lesions, and abnormal development of A1 segment in operative Anterior Cerebral Artery (ACA).
Statistical treatment
All the data were analysed on SPSS 21 software, the measurement data were described as and assessed by t-test, the count data were expressed by percentage (%) and checked by, independent factor was assessed by Man-tel-Haensze1 method with hierarchical analysis, P<0.05 suggested that there was statistically significant difference.
Results
Comparison of general data between the two groups
The Univariate analysis showed that there was statistically significant difference between CHS group and non CHS group in diabetes and postoperative hypertension (P<0.05); but not in age, sex, hypertension, hyperlipidemia, ischemic events, drinking history as well as degree of stenosis (P>0.05), as shown in Table 1.
| Item | CHS group (n=18) | Non CHS group (n=482) | χ2 | P |
|---|---|---|---|---|
| Age (y) | 68.1 ± 3.5 | 69.2 ± 3.2 | 0.347 | 0.652 |
| Male | 13 (72.22) | 417 (86.51) | 0.159 | 0.434 |
| Diabetes | 10 (55.56) | 64 (13.28) | 7.286 | 0.011 |
| Hypertension | 11 (61.11) | 296 (61.41) | 0.133 | 0.598 |
| Hyperlipidemia | 9 (50.00) | 242 (50.21) | 0.844 | 0.325 |
| Drinking history | 7 (38.89) | 189 (39.21) | 0.052 | 0.877 |
| Smoking history | 6 (33.33) | 165 (34.23) | 0.116 | 0.659 |
| Heart disease history | 5 (27.78) | 138 (28.63) | 0.552 | 0.213 |
| Stoke history | 5 (27.78) | 140 (29.05) | 1.066 | 0.142 |
| Bilateral CAS | 2 (11.11) | 38 (7.88) | 0.084 | 0.792 |
| Ischemic events | ||||
| TIA | 5 (27.78) | 135 (28.00) | 0.098 | 0.536 |
| Cerebral infarction | 9 (50.00) | 243 (50.41) | 0.065 | 0.741 |
| Postoperative hypertension | 8 (44.44) | 150 (31.12) | 9.138 | 0.016 |
| Operative stenosis degree | ||||
| 50%-69% | 7 (38.89) | 188 (39.17) | 0.019 | 0.822 |
| 70%-99% | 9 (50.00) | 243 (50.41) | 0.122 | 0.719 |
| 100% | 2 (11.11) | 51 (10.58) | 0.459 | 0.304 |
Table 1. Comparison of general data between the two groups (͞x ± s).
Comparison of imaging data of cerebral arteries
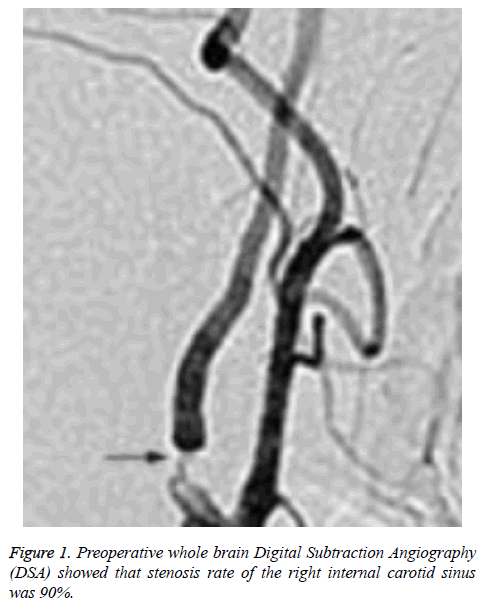
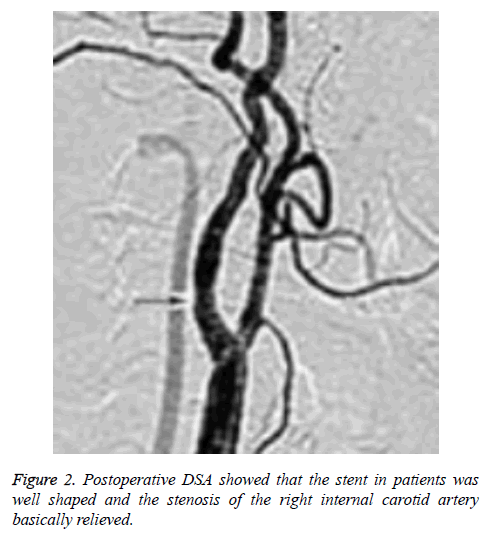
There was no statistically significant difference between CHS group and non CHS group in operative MCA occlusion, operative PCoA opening, symptomatic focus, and operative ACAA1 segment dysgenesis (P>0.05), as shown in Table 2. The relevant cerebral artery images of a typical CHS patient (a female aged 69) were shown as Figures 1 and 2.
| Item | CHS group (18 cases) | Non CHS group (514 cases) | χ2 | P |
|---|---|---|---|---|
| Operative MCA occlusion | 3 (16.67) | 86 (16.73) | 0.178 | 0.877 |
| Operative PCoA opening | 3 (16.67) | 88 (17.12) | 0.216 | 0.643 |
| Symptomatic Focus | 9 (50.00) | 253 (49.22) | 0.415 | 0.529 |
| Operative ACAA1 segment dysgenesis | 3 (16.67) | 87 (16.92) | 0.104 | 0.812 |
Table 2. Comparison of cerebral artery imaging in the two groups (n (%)).
The results of stratified analysis on risk factors for CHS
The stratified analysis showed that postoperative hypertension (OR=7.248, P<0.01) and diabetes mellitus (OR=5.906, P<0.01) were important risk factors for CHS after CASS, as shown in Table 3.
| Factor | β | OR | 95% CI | P |
|---|---|---|---|---|
| Diabetes | 1.627 | 5.906 | 1.911-15.673 | 0.001 |
| Postoperative hypertension | 2.135 | 7.248 | 2.406-22.152 | 0.001 |
Table 3. Results of stratified analysis on risk factors for CHS.
Discussion
CHS, as a deadly complication after CEA, is worth being stressed in intervention of perioperative carotid artery stenosis in Neurosurgery or Vascular Surgery. The key to the treatment of carotid stenosis is to fully understand the risk factors, characteristics and treatment of CHS [7-9]. A foreign study [10] has found that the incidence of CHS following CAS in patients with carotid stenosis ranges from 0.25% to 4.13%. Of the 500 patients selected in this study, 18 patients had CHS, accounting for 3.60%, which is basically consistent with the results of epidemiological surveys at home and abroad.
In recent years, there are many studies on the risk factors for the occurrence of CHS after CAS in patients with carotid stenosis but with no consistent conclusion. It is shown by the study that [11] the risk factors for CHS after CEA include diabetes mellitus, hypertension, coronary artery bypass grafting, low ejection fraction and blood flow factors related to operation techniques. Some studies also suggest that [12] the main risk factors for postoperative infection include diabetes, age>75 y, severe carotid artery stenosis in the operation side, history of stroke, anticoagulant use and operative MCA occlusion. In this study, the Univariate analysis showed significant difference in diabetes and the incidence of postoperative hypertension between the patients with CHS and those without (P<0.05), and the results of multivariate analysis showed that diabetes as well as postoperative hypertension were important risk factors of CHS after CAS (P<0.05).The OR value of postoperative hypertension was 7.248, suggesting that postoperative hypertension has significantly increased the incidence of CHS.
In this study, among 18 patients with CHS there were 11 cases with preoperative elevation of blood pressure, and after symptomatic treatments of decompressive craniectomy, dehydration as well as anti-hypertension, there is still 1 patient with the blood pressure more than 165 mmHg, who had intracranial hemorrhage and ended up in death. Therefore, after CAS treatment, patient’s blood pressure should be closely monitored and those with elevated pressure timely controlled [12]. We believe that in patients with vascular stenosis the elevated blood pressure will cause the increase of cerebral perfusion pressure and thus damage the blood-brain barrier, which has been destroyed before CAS, in this case the liquids in brain will continue to leak to the brain tissue gap, thereby leading to brain edema, and if there occurs rupture of blood vessel in brain, there would be subarachnoid hemorrhage or cerebral hemorrhage, thereby aggravating the patient's condition and even causing death [13]. In this study, there were 5 CHS cases with a stable blood pressure of 90-145mmHg after symptomatic treatment and the symptom of CHS disappeared completely after 2 d. Therefore, if the blood pressure can be strictly controlled within a certain range, and plus with continuously enhanced cerebral autoregulation, CHS will be much likely to disappear completely, basically in line with the results of related research [14].
At the same time, diabetes mellitus is also an important risk factor for CHS following CAS in patients with carotid stenosis. An epidemiological survey has revealed that [15] patients with diabetes were 2 times more likely to have stroke than those without. In CHS group of this study, there were 10 patients with diabetes before operation, including 2 with intracranial hemorrhage and 1 of them with death, so the mortality rate was moderately high. Related studies suggest that [16] diabetic patients are prone to cerebral vascular lesions. High blood sugar will give rise to oxidative stress reaction, which is easy to damage the vascular endothelial cells with the resulting function disorder, destroyed defense mechanism, increased gap and significantly higher vascular permeability, so patients with carotid artery stenosis and diabetes are more prone to CHS and even intracranial hemorrhage. Therefore, for patients with diabetes or history of carotid artery stenosis and history of stroke which possibly induce CHS, measures should be taken to cautiously prevent CHS after CAS treatment. We hold that following points should be paid attention for CHS prevention: (1) patients with higher blood pressure than pre-treatment in perioperative CAS period should be promptly sent to the ICU with the blood pressure strictly controlled at 90-145 mmHg; (2) the changes of patient vital signs in perioperative period should be strictly monitored followed by a detailed recording and a summary of DSA manifestations; (3) the blood glucose should be controlled in a reasonable range in diabetic patients before treatment with CAS.
However, antiplatelet drugs, age, history of stroke as well as anticoagulants were not discovered in the study as possible risk factors for CHS following CAS in patients with carotid artery stenosis, and according to the Table 2 which reveals cerebral artery imaging, there was no significant difference between the two groups of patients in operative MCA occlusion, operative PCoA opening, symptomatic focus, and operative ACAA1 segment dysgenesis (P>0.05), differing from some other research results to a certain degree. It may be related to the smaller sample size of the study. Because the incidence of CHS is moderately low in a small number of subjects, the results of this study is likely to be affected. As a result, further studies can be carried out with expanded sample size to clarify relevant risk factors.
To sum up, in elderly patients with carotid artery stenosis, those with postoperative hypertension or diabetes history are more prone to CHS following CAS with poor prognosis, which should be controlled and prevented from a variety of perspectives through intervention measures.
References
- Bakoyiannis C, Economopoulos KP, Georgopoulos S. Carotid endarterectomy versus carotid angioplasty with or without stenting for treatment of carotid artery stenosis: an updated meta-analysis of randomized controlled trials. J Int Union Angiol 2010; 29: 205-215.
- Cohen JE, Gomori JM, Itshayek E, Pikis S, Keigler G, Eichel R, Leker RR. Ischemic complications after tailored carotid artery stenting in different subpopulations with high-grade stenosis: feared but rare. J Clin Neurosci 2015; 22: 189-194.
- Brooks WH, Mcclure RR, Jones MR. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. JACC Cardiovasc Interv 2001; 38: 1589-1595.
- Wegener S, Weller M, Wong E. Hyperperfusion and changes in vasoreactivity after ischemic stroke: implications for tissue recovery. Int Symp Cerebr Blood Flow Metabol 2009; 74-75.
- Buczek J, Karlinski M, Kobayashi A. Hyperperfusion syndrome after carotid endarterectomy and carotid stenting. Cerebrovasc Dis 2013; 35: 531-537.
- Kim KH, Lee CH, Son YJ. Post-carotid endarterectomy cerebral hyperperfusion syndrome: is it preventable by strict blood pressure control? J Korean Neurosurg Soc 2013; 54: 159-163.
- Zhang XQ. Cerebrovascular stent types and hyperperfusion syndrome following stenting. J Clin Rehab Tissue Eng Res 2010; 14: 4119-4122.
- Tanaka Y, Nagaoka T, Nair G. Arterial spin labeling and dynamic susceptibility contrast CBF MRI in postischemic hyperperfusion, hypercapnia, and after mannitol injection. J Int Soc Cerebr Blood Flow Metabol 2011; 31: 1403-1411.
- Gavrilenko AV, Kravchenko AA, Kuklin AV. Prediction and risk factors of perioperative neurological complications in patients with internal carotid artery stenosis. Khirurgiia 2017; 109-112.
- Chang CH, Chang TY, Chang YJ, Huang KL, Chin SC, Ryu SJ, Yang TC, Lee TH. The role of perfusion computed tomography in the prediction of cerebral hyperperfusion syndrome. PLoS One 2011; 6: e19886.
- Ziaja D, Biolik G, Kocelak P. Neurological symptoms associated with cerebral hyperperfusion syndrome after CEA and CAS-one centre study. Eur Rev Med Pharmacol Sci 2014; 18: 1176-1180.
- Oshida S, Ogasawara K, Saura H, Yoshida K, Fujiwara S, Kojima D, Kobayashi M, Yoshida K, Kubo Y, Ogawa A. Does preoperative measurement of cerebral blood flow with acetazolamide challenge in addition to preoperative measurement of cerebral blood flow at the resting state increase the predictive accuracy of development of cerebral hyperperfusion after carotid endarterectomy? Results from 500 cases with brain perfusion single-photon emission computed tomography study. Neurol Med Chir (Tokyo) 2015; 55: 141-148.
- Sonobe S, Fujimura M, Endo H. Subarachnoid hemorrhage due to ruptured posterior cerebral artery aneurysm simultaneously associated with multiple remote intracerebral hemorrhages-case report. Neurologia Medico Chirurgica 2011; 51: 836-838.
- Ivens S, Gabriel S, Greenberg G. Blood-brain barrier breakdown as a novel mechanism underlying cerebral hyperperfusion syndrome. J Neurol 2010; 257: 615-620.
- Dieter RS. Postcarotid endarterectomy hyperfusion or reperfusion syndrome. Stroke 2005; 36: 706-707.
- Hay CJ, Kalra PA. Review: renovascular disease in diabetic patients. Br J Diab Vasc Dis 2002; 2: 91-95.