Research Article - Biomedical Research (2017) Volume 28, Issue 7
Analysis of the inhibitory effect of safflower polysaccharide on HT29 colorectal cancer cell proliferation and its relevant mechanism
Ai Liang, Zhu Jianghong, Zhang Taijun, Li Xiaoqing, Zhang Qiong and Cheng Jun*Discipline of Pharmacology, School of Pharmacy, University of the Western Cape, Bellville 7535, South Africa
- *Corresponding Author:
- Cheng Jun
Department of Oncology
Chongqing Hospital of Traditional Chinese Medicine, Chongqing, PR China
Accepted date: November 30, 2016
Abstract
Objective: To explore the inhibitory effect of safflower polysaccharide on HT29 colorectal cancer cell proliferation and its relevant mechanism.
Method: MTT assay was used to detect the inhibitory effect of safflower polysaccharide on HT29 colorectal cancer cell proliferation. Morphology of colorectal cancer cell apoptosis was observed. Cell cycle and apoptosis was assayed using Annexin V and PI double staining flow cytometry. Caspase-3 protein expression was detected by Western blot.
Results: The inhibitory rate in every experimental group with different concentration was significantly higher than that in control group (p<0.05). The inhibitory rate was more significant along with the increasing concentration of the drug dose-dependently. IC50=201.908 mg/L. The concentration to HT29 cells was respectively 160 mg/L, 320 mg/L and 640 mg/L in experimental group. The HT29 cell cycle was affected. The main block was in HT29 cells G2/M phase, S phase. The apoptosis rate in experimental group with 160 mg/L, 320 mg/L and 640 mg/L was significantly higher than control group dosedependently (p<0.05). Caspase-3 protein expression in experimental group with 160 mg/L, 320 mg/L and 640 mg/L was significantly higher than control group (p<0.05). The expression increased along with the increasing concentration. This demonstrated that safflower polysaccharide could significantly upregulate Caspase-3 protein.
Conclusion: Safflower polysaccharide could significantly inhibit HT29 colorectal cancer cell. The mechanism of it inducing HT29 cell apoptosis might have correlation with blocking cells in G2/M phase, S phase, and up-regulating Caspases-3 protein expression.
Keywords
Safflower polysaccharide, HT29 colorectal cancer cell, proliferation, inhibition, mechanism
Introduction
Colorectal cancer is a common malignancy. Epidemiological studies found that with the modern lifestyle changes and increasing population aging process, the incidence of colon cancer showed an increasing trend [1-6]. It has now become the third malignant tumor after lung and breast cancer, which is a serious threat to people's lives. Safflower is a traditional Chinese medicine, with the effect of promoting blood circulation, dispersing blood stasis and analgesic. Modern pharmacological studies have shown it had anti-tumor and improving immunity effect, but its exact mechanism of antitumor is not very clear [7-9]. In this study, HT29 cells from colon cancer tissues were selected as the experimental study. The aim of this study was to investigate the inhibitory effect of safflower polysaccharide on HT29 cell proliferation and its related mechanism. Further studies on HT29 cell lines were needed for a certain experimental basis.
Materials and Methods
Experimental cells and experimental drugs
HT29 human colon cancer cells ( purchased from the National Laboratory of Biology Treatment Sichuan University). Safflower (purchased from Zhongnuo Biotechnology Co., Ltd., Shanxi), safflower polysaccharide was extracted by water extraction and alcohol precipitation. The identification of purchased drug was in line with the 2010 edition of "Chinese Pharmacopoeia" regulations.
Reagents and instruments
DMEM medium (Gibco Company, US), 0.25% trypsin (Hyclone, US), dimethyl sulfoxide (DMSO, Kermel Chemical Reagent Co., Ltd., Tianjin), Annexin V-FITC apoptosis detection kit (KGI Biological Production Co., Ltd., Nanjing). Microplate reader (Thermo Company, US), FACS Canto II flow cytometer (BD Biosciences, USA), inverted microscope (Olympus, Japan).
Grouping
The cells were divided into control group and experimental group. Cells in experimental group were divided into 6 groups according to the drug concentration, namely 20 mg/L, 40 mg/L, 80 mg/L, 160 mg/L, 320 mg/L, 640 mg/L.
Methods
HT29 colon carcinoma cell culture: HT29 colon cancer cells were cultured using 10% DMEM medium containing fetal bovine serum seeded to culture flasks and placed in 37°C, 5% CO2 incubator. The medium was changed every day. Cells were subcultured every 2 to 3 days. Cells in logarithmic growth phase were used for experiments.
Detection of the inhibitory effect of safflower polysaccharide on HT29 colorectal cancer cell proliferation using MTT assay: The above HT29 colon cancer cells in logarithmic growth phase were seeded on to a 96 well plate at 2 × 103 cells/well. After incubation for 24 h, the sample culture medium was added to each group with five wells. After cultured for 48h, 20 μL 5 mg/mL MTT solution was added to each well. After 4 h of incubation at 37°C, 5% CO2 incubator, the supernatant was discarded. 150 μL of DMSO solution was added to each well to dissolve the purple crystal precipitate, with oscillation for 10min. The above processes were carried out for 3 times. The optical density (OD) was measured using a microplate reader at 570 nm to compute cell survival rate and median lethal concentration (IC50). Cell survival rate (%)=A sample-A blank/(A control-A blank) × 100%. A sample was the OD after different concentration of safflower polysaccharide was added. A control was the optical density of the negative control whole cells. A blank was the optical density of the blank control group.
Morphological examination of colon cancer cell apoptosis: The progress of cell apoptosis was assessed using the nuclear chromatin morphology as an index. Hochest 33258/PI double staining was used to identify apoptotic and necrotic cells. The HT29colon carcinoma cells to be seeded in 6-well plates at 3 × 105. After incubation for 24 h, 160 mg/L, 320 mg/L, 640 mg/L safflower polysaccharide culture medium was added, then placed in a 37°C, 5% CO2 incubator. After incubation 48 h, Hochest 33258 stock solution was added to a final concentration of 1 μg/mL, and then incubated at 37°C, 5% CO2 incubator for 10 min. Then it was centrifuged at 1000 r/min for 5 min. Staining solution was discarded. 1 mL 5 μg/mL PI staining solution was added to stain for 15 min at 4°C away from light. The cells were collected and coated on a slide to 10 μL drop anti-fluorescein mounting liquid. The coverslip was covered immediately. Cells were observed under an inverted fluorescence microscope. Cell morphology between the groups was compared.
Detection of cell cycle and apoptosis using Annexin V and PI double staining flow cytometry: HT29 cells were seeded in 6-well plates at 3 × 105 for 24 h of incubation. Then 160 mg/L, 320 mg/L, 640 mg/L safflower polysaccharide culture medium was added, then placed in a 37°C, 5% CO2 incubator for 48 h. The supernatant was discarded, and trypsin was used to digest and cell collected. Cells were washed with cold PBS three times and centrifuged at 1200 r/min for 5 min. Cells were resuspended, counted, to make each tube reach 1 × 106 ~ 5 × 106/mL. Cell pellet was collected by centrifugation, and then resuspended in 200 μL binding buffer. Annexin V-FITC 5 μL was added and then 5 μL PI for 10 min of reaction at room temperature away from light. 300 μL binding buffer was used to mix. Flow cytometry was used for detection.
Detection of Caspase-3 protein by Western blot: The above cultured HT29 cells and collected HT29 cells that treated by 160 mg/L, 320 mg/L, 640 mg/L safflower polysaccharide for 48 h were digested by trypsin and centrifuged. Total proteins were extracted using PIRA lysate. BCA assay was sued for detecting protein concentration. After SDS- polyacrylamide gel electrophoresis, it was semi-dry blotted to a PVDF membrane and sealed in 5% skim milk for 1.5 h. Caspase-3 primary antibody was added at 4°C overnight. Diluted secondary antibody was added after washing the membrane (1: 500) and incubated at room temperature for 1h. ECL coloration was used for exposure. Gel imaging system was sued for scan analysis. β-actin protein expression were detected, as an internal reference.
Statistical analysis
SPSS 22.0 statistical software was used to process data. Measurement data were represented as (͞x ± s). LSD-t test was used for pairwise comparison within groups. ANOVA was used for comparison between groups. P<0.05 indicates a statistically significant difference.
Results
Detection of the inhibitory effect of safflower polysaccharide on HT29 colorectal cancer cell proliferation using MTT assay
As illustrated in Table 1. The inhibitory rate in every experimental group with different concentration was significantly higher than that in control group (p<0.05). The inhibitory rate was more significant along with the increasing concentration of the drug dose-dependently. IC50=201.908 mg/L.
| Groups | n | Inhibitory rate (%) |
|---|---|---|
| Control group | 5 | 0.00 ± 0.00 |
| Experimental groups | ||
| 20mg/L | 5 | 9.48 ± 2.34* |
| 40mg/L | 5 | 18.79 ± 3.49* |
| 80mg/L | 5 | 30.14 ± 4.78* |
| 160mg/L | 5 | 43.29 ± 5.42* |
| 320mg/L | 5 | 58.82 ± 8.93* |
| 640mg/L | 5 | 74.39 ± 11.34* |
Table 1. Detection of the inhibitory effect of safflower polysaccharide on HT29 colorectal cancer cell proliferation using MTT assay (͞x ± s).
Morphology test results of colon cancer cell apoptosis
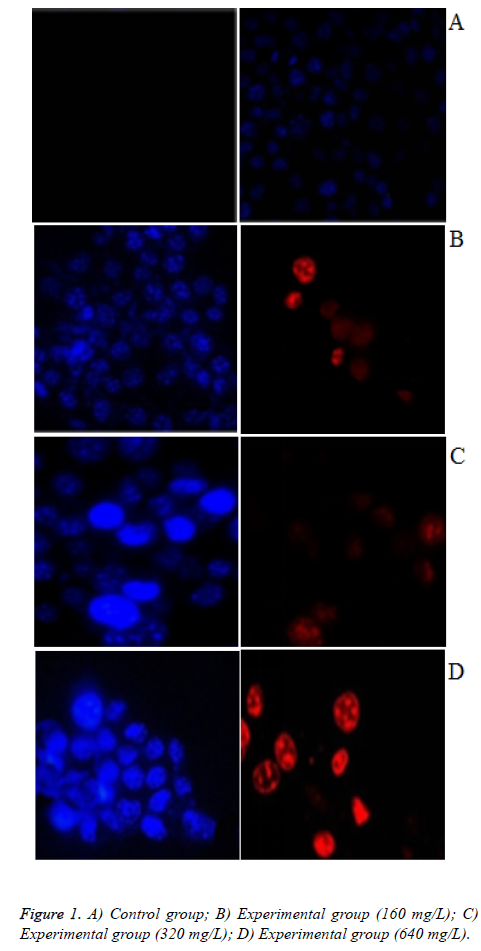
As illustrated in Figures 1A-1D, According to IC50=201.908 mg/L, we selected the concentration of safflower polysaccharide at 160 mg/L, 320 mg/L, 640 mg/L. PI cannot pass through normal cell, Hoechst is a fluorescent dye with membrane permeability. Therefore, when in late apoptosis or necrosis, cell membrane is damaged, and then the cells can be dyed red by PI. Cells in control group were not stained red fluorescence, with the increasing concentration of safflower polysaccharide, some cells were colored by PI. In late apoptosis and necrosis, when safflower polysaccharide concentration increased to 640 mg/L, blue and red light appear superimposed. The chromatin of nuclei was highly coagulated and marginalized, with obvious apoptotic bodies, belonging to late apoptosis.
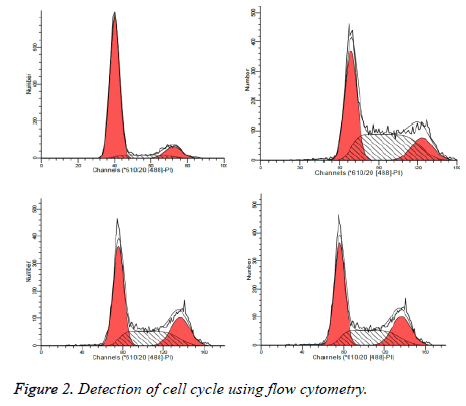
Detection of cell cycle using flow cytometry
As illustrated in Table 2 and Figure 2, treated by safflower polysaccharide at various concentrations, cell cycle of the HT29 was affected, mainly blocked at G2 / M phase, S phase.
| Groups | G0-G1phase | G2-M phase | S phase |
|---|---|---|---|
| Control group | 77.83 ± 5.42 | 12.16 ± 4.13 | 10.01 ± 2.14 |
| Experimental groups | |||
| 160 mg/L | 39.65 ± 5.23* | 14.49 ± 4.07 | 44.86 ± 5.24* |
| 320 mg/L | 44.61 ± 3.63* | 17.30 ± 4.58* | 38.09 ± 3.97* |
| 640 mg/L | 45.49 ± 4.10* | 19.79 ± 3.65* | 34.72 ± 4.25* |
Table 2. Detection of cell cycle using flow cytometry (͞x ± s).
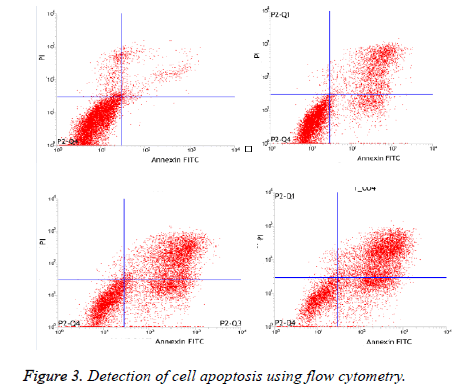
Detection of cell apoptosis using flow cytometry
As illustrated in Table 3 and Figure 3, The apoptosis rate in experimental group with 160mg/L, 320mg/L and 640mg/L was significantly higher than control group dose-dependently (p<0.05).
| Groups | Apoptosis rate (%) |
|---|---|
| Control group | 6.92 ± 1.32 |
| Experimental groups | |
| 160 mg/L | 34.99 ± 4.25* |
| 320 mg/L | 61.89 ± 6.78* |
| 640 mg/L | 71.91 ± 9.32* |
Table 3. Detection of cell apoptosis using flow cytometry (͞x ± s).
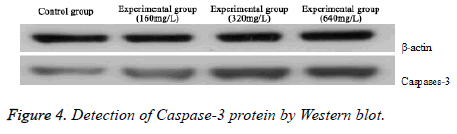
Detection of Caspase-3 protein by Western blot
As illustrated in Figure 4, Caspase-3 protein expression at different concentration safflower polysaccharide was significantly higher than that in control group. The expression increased along with the increasing concentration, demonstrating that safflower polysaccharide could significantly up-regulate Caspase-3 protein.
Discussion
At present, the clinical treatment of colon cancer was comprehensive treatment based on surgery, with 60% to 70% of surgical resection rate. But recurrence and metastasis is a major cause of mortality after surgery, and most chemotherapy drugs will bring a lot of side effect [10,11]. Recent studies found that traditional Chinese medicine had achieved good results in anti-tumor, with little side effect. Safflower, also known as gold saffron, Du safflower, thorn safflower, is a traditional Chinese medicine, with the effect of promoting blood circulation, dispersing blood stasis and warming the channels to relieve pain. Pharmacological studies have shown it to have anti-cancer, sterilization and other effects, with extensive clinical applications [12]. Yan et al. [13] reported that safflower polysaccharide could inhibit the expression of cell cycle-related genes, and induced liver SMMC-7721 cell blocked, so as to achieve anti-tumor effects. In this study, the inhibitory rate in every experimental group with different concentration was significantly higher than that in control group. The inhibitory rate was more significant along with the increasing concentration of the drug dose-dependently. This demonstrated that safflower polysaccharide had significant tumor inhibitory effect.
Cancers are a class of cell cycle diseases, most of them are uncontrolled proliferative diseases, with a number of external regulation factors including growth factors, drugs, nutrients etc., affecting cell cycle progression, and further inducing cell proliferation, cell cycle arrest or apoptosis [14,15]. Cell cycle, the basic process of tumor cell life activities, is divided into four periods: G1 phase, S phase, G2 phase and M phase. Wherein the G1 and G2 phases are respectively the early and late DNA synthesis, S phase is DNA synthesis phase [16]. Apoptosis of tumor cells, also known as programmed cell death, is an important part of cell life cycle which is regulated by cell-intrinsic gene coding. Cell suicide is promoted by active biochemical processes. The process is a process of cellautonomous death regulated by multiple genes [17]. Apoptosis is an important part of body regulating growth and development and maintaining a stable internal environment. Treated by safflower polysaccharide at various concentrations, cell cycle of the HT29 was affected, mainly blocked at G2/M phase, S phase. The apoptosis rate in experimental group with 160mg/L, 320mg/L and 640 mg/L was significantly higher than control group dose-dependently. In this study, we have shown that G2/M phase and S phase of HT29 cells are mainly blocked by the preliminary study, but we need further study in the follow-up to provide a reliable reference value for the effect of safflower polysaccharide on cell cycle of HT29 tumor cells.
Caspase family is a large class of apoptosis regulating genes. As the effector molecule of apoptosis, Caspase-3 is one of the key hydrolysis protease presented in downstream of the apoptotic pathway, known as apoptosis "performer" [18,19]. The activation of Caspase-3 expression can promote cytoskeletal damage, destruct some proteases, eventually leading to characteristic DNA fragmentation, promoting apoptosis [20,21]. In this study, caspase-3 protein expression at different concentration safflower polysaccharide was significantly higher than that in control group. The expression increased along with the increasing concentration, demonstrating that safflower polysaccharide could significantly up-regulate Caspase-3 protein.
In summary, safflower polysaccharide could significantly inhibit HT29 colorectal cancer cell. The mechanism of it inducing HT29 cell apoptosis might have correlation with blocking cells in G2/M phase, S phase, and up-regulating Caspases-3 protein expression.
Acknowledgement
Fund support: This work was supported by a grant from the General designed of Science and Technology themselves for Foundation of Chongqing (No. Cstc2014jcyjA10079).
References
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014; 64: 104-117.
- Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012; 61: 847-854.
- Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687-696.
- Li Z, Meng J, Xu TJ, Qin XY, Zhou XD. Sodium selenite induces apoptosis in colon cancer cells via Bax-dependent mitochondrial pathway. Eur Rev Med Pharmacol Sci 2013; 17: 2166-2171.
- Li HG, Zhao LH, Bao XB, Sun PC, Zhai BP. Meta-analysis of the differentially expressed colorectal cancer-related microRNA expression profiles. Eur Rev Med Pharmacol Sci 2014; 18: 2048-2057.
- Sung JJ, Lau JY, Goh KL, Leung WK; Asia Pacific Working Group on Colorectal Cancer. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol 2005; 6: 871-876.
- Si N, Wang J, Xu Y. Inductive effect of hydroxyl safflower yellow-A on apoptosis in abnormal HUVEC via the mitochondrial pathway. J Trad Chin Med Sci 2015.
- Ren A N, Lu Y, Zou Y. Separation, purification and preliminary structure analysis of acidic polysaccharides from safflower. Eur Food Res Technol 2013; 237: 449-455.
- Luo Z, Zeng H, Ye Y, Liu L, Li S, Zhang J, Luo R. Safflower polysaccharide inhibits the proliferation and metastasis of MCF-7 breast cancer cell. Mol Med Rep 2015; 11: 4611-4616.
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013; 14: 1208-1215.
- Vlug MS, Bartels SA, Wind J, Ubbink DT, Hollmann MW, Bemelman WA; Collaborative LAFA Study Group. Which fast track elements predict early recovery after colon cancer surgery. Colorectal Dis 2012; 14: 1001-1008.
- Sheng-yan XI ,Qian Z, Chun W, Jian-jun Z, Xue-min G. Discussion of Safflower Inhibiting Tumor in Application and Its Mechanism of Action. Chin Arch Trad Chin Med 2008; 9: 032.
- Yan X, Chen J, Wang M. Determination of Gallic Acid on In-vitro Toxicity of Two Kinds of Cells by MTT. Inform Trad Chin Med 2012; 2: 046.
- Polycarpou E, Meira LB, Carrington S, Tyrrell E, Modjtahedi H, Carew MA. Resveratrol 3-O-d-glucuronide and resveratrol 4'-O-d-glucuronide inhibit colon cancer cell growth: Evidence for a role of A3 adenosine receptors, cyclin D1 depletion, and G1 cell cycle arrest. Mol Nutr Food Res 2013; 57: 1708-1717.
- Suboj P, Babykutty S, Srinivas P, Gopala S. Aloe emodin induces G2/M cell cycle arrest and apoptosis via activation of caspase-6 in human colon cancer cells. Pharmacol 2012; 89: 91-98.
- Darzynkiewicz Z, Zhao H. Cell cycle analysis by flow cytometry. eLS, 2014.
- Vaux DL. Another twist in the on and off affair between cell suicide and inflammation. Cell Death Differ 2013; 20: 974-975.
- Liu P, Liang S, Yao N. Programmed cell death of secretory cavity cells in fruits of Citrus grandis cv. Tomentosa is associated with activation of caspase 3-like protease. Trees 2012; 26: 1821-1835.
- Simpson KL, Cawthorne C, Zhou C, Hodgkinson CL, Walker MJ, Trapani F, Kadirvel M, Brown G, Dawson MJ, MacFarlane M, Williams KJ, Whetton AD, Dive C. A caspase-3 ‘death-switch’in colorectal cancer cells for induced and synchronous tumor apoptosis in vitro and in vivo facilitates the development of minimally invasive cell death biomarkers. Cell Death Dis 2013; 4: e613.
- Lee JS, Jung WK, Jeong MH, Yoon TR, Kim HK. Sanguinarine induces apoptosis of HT-29 human colon cancer cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent pathway. Int J Toxicol 2012; 31: 70-77.
- Tan BL, Norhaizan ME, Huynh K, Heshu SR, Yeap SK, Hazilawati H, Roselina K. Water extract of brewers’ rice induces apoptosis in human colorectal cancer cells via activation of caspase-3 and caspase-8 and downregulates the Wnt/ß-catenin downstream signaling pathway in brewers’ rice-treated rats with azoxymethane-induced colon carcinogenesis. BMC Complement Altern Med 2015; 15: 1.