Short Article - Journal of Brain and Neurology (2018) Volume 1, Issue 1
Analysis of the curative effect of a new decompressive Craniectomy on the treatment of severe Craniocerebral injury
Qianlei Liang1, Xin Chen2, Dehua Liu1, Yueting Gao3, Yongchuan Guo1*
1Department of Neurosurgery, China-Japan Union Hospital, Jilin University, Changchun 130033, China
2Radiotherapy Center, Shaanxi Provincial People's Hospital, Xi'an 710068, Shaanxi, China
3Operating Room, China-Japan Union Hospital, Jilin University, Changchun 130033, China
- *Corresponding Author:
- Yongchuan Guo
Department of Neurosurgery, China-Japan Union Hospital of Jilin University, 126 Xiantai Street, Changchun, Jilin Province, 130033, China
Tel: +86 13504310682
E-mail: guoyongchuan@163.com
Accepted on January 16, 2018
Citation: Liang Q, Chen X, Liu D, et al. Analysis of the curative effect of a new decompressive craniectomy on the treatment of severe craniocerebral injury. J Brain Neurol 2018;2(1):1-7..
Abstract
At present, severe and extra severe craniocerebral injury are still the difficulties in clinical neurosurgery, with a high mortality rate of 30%-50% and a high disability rate. Complications such as cerebral contusion and laceration, hydrocephalus, and diffuse brain swelling are often developed and then induce rises of malignant intracranial pressure, which is the main cause of mortality. For severe and extra severe craniocerebral injury, conservative treatments are often ineffective, while decompressive craniectomy is the key to cure patients. Neurosurgeons hold different views on the removing scope of the bone flap and its merits and demerits. We have designed a new decompressive craniectomy based on years of treatment of severe and extra severe craniocerebral injuries.
Keywords
Severe craniocerebral injury, New decompressive craniectomy, Standard decompressive craniectomy.
Introduction
At present, severe and extra severe craniocerebral injury are still the difficulties in clinical neurosurgery, with a high mortality rate of 30%-50% and a high disability rate [1]. Complications such as cerebral contusion and laceration, hydrocephalus, and diffuse brain swelling are often developed and then induce rises of malignant intracranial pressure, which is the main cause of mortality [2-4]. For severe and extra severe craniocerebral injury, conservative treatments are often ineffective, while decompressive craniectomy is the key to cure patients. Neurosurgeons hold different views on the removing scope of the bone flap and its merits and demerits [5]. We have designed a new decompressive craniectomy based on years of treatment of severe and extra severe craniocerebral injuries.
Subjects and Methods
The objects of this study are patients with severe and extra severe craniocerebral injury who received decompressive craniectomy operations from December, 2012 to March, 2016. Among them 36 are males and 20 are females, aged from 18 to 70; 30 were injured by road accidents, 10 by falling from high places, 9 by blows and 7 by tumbles. The 56 patients were grouped randomly by flipping coins into a test group of 26 cases to receive the new decompressive craniectomy and a control group of 30 cases to receive the standard decompressive craniectomy. The differences of GCS scores, gender and age between the two groups when they were admitted into the hospital have no statistical significance (P>0.05).
The inclusion criteria of patients were:
1) explicit head injury, no evident trauma history of the chest, abdomen, limbs, etc. that threatened the life of the patients; 2) patients with severe and extra severe craniocerebral injury with a GCS scores of 3 to 8; if a patient is in a deep coma, with bilateral or unilateral mydriasis, he/she is suffering from cerebral hernia; 3) patients who, according to the head CT scan, suffered from multiple intracranial cerebral contusion, intracranial hematoma, epidural hematoma, subdural hematoma, diffuse brain swelling after injuries, evident compression or disappearance of the cerebral cistern such as the ambient cistern and the lateral fissure cistern, evident middle line shift, or evident compression of the lateral ventricles.
The exclusion criteria of patients are:
1) patients with severe and extra severe craniocerebral injury whose bilateral pupils were in continuing dilation; 2) patients with severe and extra severe craniocerebral injury who had respiratory dysfunction and continuing decreasing oxygen saturation; 3) patients with Cushing's reaction.
Surgical procedures
The test group received the new decompressive craniectomy and the control group received the standard decompressive craniectomy.
New decompressive craniectomy
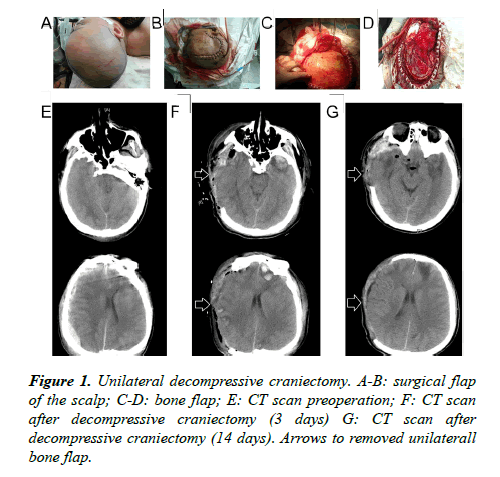
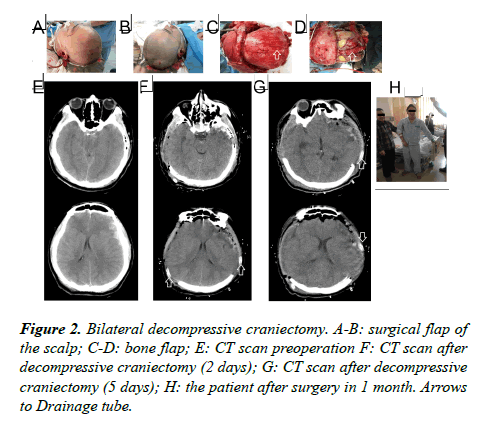
Surgical flaps: Starting from 1 cm ahead of the zygomatic arch upper tragus, extending upward from the back of the auricle to the parietal tuber, passing the middle line of the parietal bone, extending forward to the contralateral forehead within the hairline, then cutting 1-2 cm besides the middle line (Figure 1); the flap incision of bilateral decompressive craniectomy was 2 cm behind the convergence of unilateral flap incisions in the coronal suture (approximately a "W" shaped incision) (Figure 2).
Bone flap: A free bone flap was applied; the front of the removing unilateral bone flap was flush with the anterior skull base; the inside needed to reach the middle line as far as possible, the back reaching the parietal tuber and the outer or lower side reaching the middle skull base; the interior sphenoid ridge till the superior orbital fissure was completely removed; the greater wing of the sphenoid bone was partially resected and the squama temporalis completely resected, so that the anterior cranial fossa was flush with the middle cranial fossa, and the anterior and middle skull base would be completely decompressed (Figure 1); the range of the removing bilateral bone flap is the same with the range of the removing unilateral bone flap on both sides; the size of the intermediate beam bone was about 2-3 cm (Figure 2).
Cutting the dura mater: The dura mater was cut from the front temporal lobe in the shape of a claw. The frontal lobe, the temporal lobe, the parietal lobe, the anterior cranial fossa, the middle cranial fossa and the intracranial hematoma could be fully exposed.
All patients were elevated by the head at an angle of 30 degrees. Their tracheas were cut open and the ventilators were used for breathing assistance. Under mild hypothermia and brain protection, nourishing brain cells were given and oxygen free radicals were removed by drugs. Dehydration drugs were applied according to the intracranial pressure of patients indicated by the intracranial pressure monitor; blood sugar level and electrolyte balance were maintained. The control group received the same treatment with the test group.
Observational indexes
• ICP shown indirectly by changes of the ambient cistern on the head CT. The intracranial pressure and severity of the illness were known through the morphological changes (normal, compression, abolition) of the ambient cistern on head CT in preoperative and postoperative 3d, 7d [5].
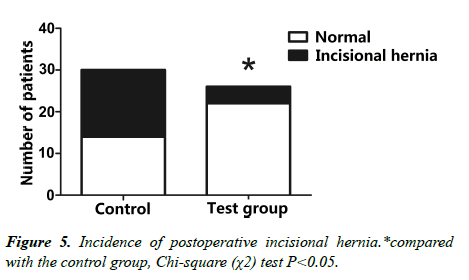
• The incidence of intraoperative and postoperative complications of emergency, such as intraoperative acute cephalocele and postoperative incision hernia.
• GCS scores in preoperative and postoperative 1d, 3d, 7d.
• ICP changes indicated by the intracranial pressure monitor in postoperative 3d, 5d, 7d.
• GOS scores in postoperative 3 months, 6 months and 12 months.
Statistical analysis
In this study, the data were statistically analyzed using SPSS17.0 software package. The data of intraoperative cephalocele, postoperative incision hernia and postoperative GOS scores in 3 months, 6 months and 12 months were tested by chi-square (χ2); GCS scores in preoperative and postoperative 1d, 3d, 7d were represented as mean ± standard deviation (x̅ ± s). P<0.05 was considered statistically significant.
Results
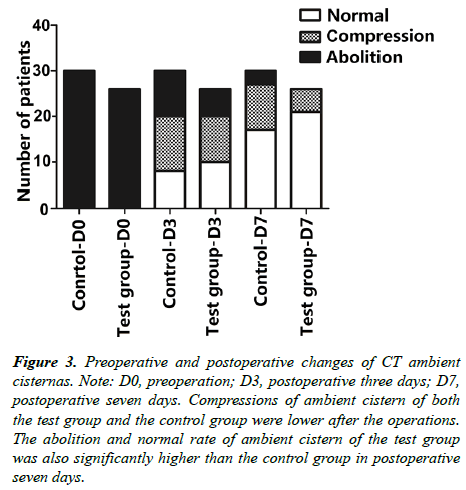
Statistics of ambient cistern changes on preoperative and postoperative head CT of patients receiving new decompressive craniectomy and standard decompressive craniectomy (Figure 3).
Figure 3: Preoperative and postoperative changes of CT ambient cisternas. Note: D0, preoperation; D3, postoperative three days; D7, postoperative seven days. Compressions of ambient cistern of both the test group and the control group were lower after the operations. The abolition and normal rate of ambient cistern of the test group was also significantly higher than the control group in postoperative seven days.
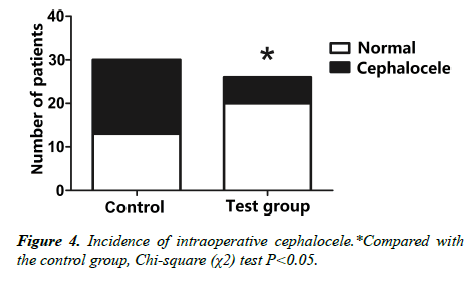
2. Comparison of two groups of patients undergoing cephalocele (Figure 4).
3. The comparative analysis of postoperative incision hernia of the two groups (Figure 5).
4. Comparative statistics of GCS scores in preoperative and postoperative 1d, 3d, 7d of the two groups of patients (Table 1).`
| Group | N | Preoperative | 1d | 3d | 7d |
|---|---|---|---|---|---|
| Test | 30 | 4.6 ± 1.4 | 4.7 ± 1.6 | 5.6 ± 1.4 | 6.9 ± 1.5 |
| Control | 26 | 4.4 + 1.2 | 5.6 ± 1.2* | 7.0 ± 1.1* | 8.9 ± 1.3* |
*Compared with the control group, Chi-square (χ2) test P<0.05.
Table 1: Preoperative and postoperative GCS scores of the patient (x̅ ± s).
5. Comparative statistics of intracranial pressure (ICP) in postoperative 1d, 3d, 7d of the two groups of patients (Table 2).
| Group | N | Preoperative | 3 d | 7 d |
|---|---|---|---|---|
| Test | 30 | 247.00 ± 41.95 | 222.67 ± 35.23 | 192.67 ± 29.35 |
| Control | 26 | 208.08 ± 25.77* | 189.62 ± 23.06* | 163.85 ± 22.82* |
*Compared with the control group, Chi-square (χ2) test P<0.05.
Table 2: Postoperative ICP of patients (x̅ ± s).
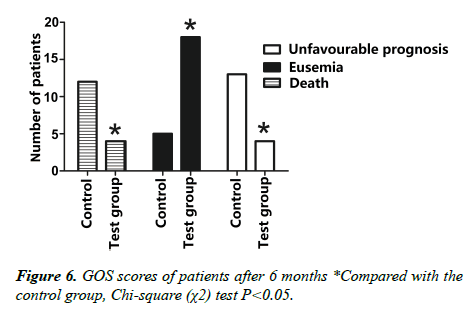
6. Comparative statistics of GOS scores in postoperative 3 months of the two groups of patients (Table 3, Figure 6).
| Group | N | mild disability | Moderate disability |
Severe disability |
Vegetative State |
death |
|---|---|---|---|---|---|---|
| Test | 30 | 3* | 2* | 6 | 7 | 12 |
| Control | 26 | 8 | 10 | 2 | 2 | 4 |
*Compared with the control group, Chi-square (χ2) test P<0.05.
Table 3: Number of patients with different GOS scores after 3 months.
Statistical analysis was made according to: eusemia (grade IV, moderate disability; grade V, good recovery (mild disability)), unfavorable prognosis (grade II, vegetative state; grade III, severe disability; grade IV), and Grade I death.
7. Comparative statistics of GOS scores in postoperative 6 months of the two groups of patients (Table 4).
| Group | N | mild disability | Moderate disability |
Severe disability |
Vegetative State |
death |
|---|---|---|---|---|---|---|
| Test | 30 | 3* | 3* | 5 | 6 | 13 |
| Control | 26 | 9 | 9 | 2 | 2 | 4 |
*Compared with the control group, Chi-square (χ2) test P<0.05.
Table 4: Number of patient GOS with different scores after 6 months.
8. Comparative statistics of GOS scores in postoperative 12 months of the two groups of patients (Table 5).
| Group | N | mild disability | Moderate disability |
Severe disability |
Vegetative State |
death |
|---|---|---|---|---|---|---|
| Test | 30 | 3* | 4* | 4 | 6 | 13 |
| Control | 26 | 9 | 9 | 2 | 1 | 5 |
*Compared with the control group, Chi-square (χ2) test P<0.05.
Table 5: number of patients with different GOS scores after 12 months.
Indexes such as the GCS scores, the intracranial pressure, the middle line shift, etc. have all shown significant improvement after the new decompressive craniectomy in 7 days. The improvement is more obvious than the standard decompressive craniectomy (P<0.05). The incidence of cephalocele and incision hernia in the test group are significantly lower than in the control group (P<0.05). It was discovered in a follow-up visit, 12 months after the operations, that in the test group, there were 9 cases of good recovery (mild disability), 9 medium disability, 2 severe disability, 1 vegetative state and 5 deaths. While in the control group, there were 3 cases of good recovery (mild disability), 4 medium disability, 4 severe disability, 6 vegetative state and 13 deaths; the rate of favorable prognosis of the test group (69.2% , 18/26) is significantly higher than the control group (23.3%,7/30); the mortality rate of the test group (19.2%,5/26) is significant lower than the control group (43.3%, 13/30).
Discussion
Severe and extra severe craniocerebral injuries are serious and complicated illnesses with rapid progression, poor prognosis and high disability and mortality rate; post-traumatic cephaledema and malignant high intracranial pressure are the main influencing factors of prognosis, while cerebral ischemia, hypoxia are the main reasons for secondary brain injury, interacting with other influencing factors of secondary brain injury (eg: BBB damage, disorder of cerebrospinal fluid circulation, etc.), further exacerbating brain injury and cephaledema, and affecting the prognosis [6]. For patients with severe and extra severe craniocerebral injury, conservative treatment is ineffective. Decompressive craniectomy is the key to the treatment of patients.
The necessity of decompressive craniectomy for severe and extra severe craniocerebral injury
In order to relieve damage to the brain caused by malignant high intracranial pressure of severe traumatic brain injuries, currently the main STBI treatment options include conservative treatment, surgery, and other special treatment (such as mild hypothermia, brain protection, etc.). Conservative treatment is rather ineffective, among which decompressive craniectomy is the key to the cure [4]; surgical indications of decompressive craniectomy for patients with severe and extra severe craniocerebral injury: (1) patients with GCS<8, in a coma or a deep coma, with one side of pupils dilated and cerebral hernia; (2) diffuse brain injury (cerebral axonal injury, brain swelling), head CT showing significant compression or disappearance of the ambient cistern, and the disappearance of the sulcus and the gyrus [7,8]; (3) patients with bilateral hematoma, middle line shift>5 mm; patients with unilateral hematoma, middle line shift>10 mm, significant compression or occlusion of ipsilateral or bilateral paraceles; (4) subdural hematoma combined with brain contusions, significant decrease of GCS scores (5) supratentorial hematoma>30 ml or infratentorial hematoma>10 ml; (6) rupture of superior sagittal sinus and transverse sinus, causing massive haemorrhage or intracranial hematoma [9-11].
Comparison of the advantages and disadvantages of the new decompressive craniectomy and standard decompressive craniectomy
In clinical practice, the standard decompressive craniectomy is still inadequate in the following aspects: 1) damage to the combined temporal contusion, the Labbe vein, the transverse sinus, or the superior sagittal sinus, which are often found rather difficult to deal with in the application of standard decompressive craniectomy. Sometimes a "T" shaped incision needs to be added to expand the bone window backward. This not only increases operation time, the intracranial pressure not having been mitigated timely, but also veins on the surface and depth of the brain such as Labbe vein, the sagittal sinus, transverse sinus and other veins remain under pressure, affecting cerebral venous reflux, leading to sustained brain ischemia, hypoxia, a corresponding change in the pathophysiology of intracranial hypertension, and an increasingly malignant high cranial pressure [12]; 2)Since the brain tissue is in a swelling state after the brain injury, and the size of the bone window is definite when applying standard decompression craniectomy, the thin layer of potential subdural hematoma in the skull base cistern and other places cannot be cleared timely, causing cerebrospinal fluid circulation disorder, increasing the incidence of hydrocephalus, and worsening the patient's condition and prognosis; 3)Since the bone window is small in the operation of standard decompressive craniectomy, the temporal lobe, the frontal lobe, and the parietal lobe are not decompressed adequately, leading to cerebral ischemia and hypoxia, disorder of cerebrospinal fluid circulation, obvious secondary swelling of brain tissue after the operation, and incision hernia, further exacerbating the circulation disorder of cerebrospinal fluid and blood, softening the brain tissue in the decompressed area and causing vascular necrosis, increasing intracranial pressure, the rate of cerebral infarction and vascular occlusion, later causing cerebromalacia and cerebral atrophy of surviving patients, and increasing the incidence of epilepsy, and worsening prognostic [13]; 4) After decompressive craniectomy for severe brain injury, changes of electrolytes and osmotic pressure in extracellular fluid and blood cause intracellular edema, also known as osmotic pressure brain edema. Since brain swelling is still evident after standard decompressive craniectomy, the dose of dehydration drugs such as mannitol increases significantly, which is reported to cause up to 20% impairment of renal function. Large doses of mannitol may lead to heart and kidney damage, increase the incidence of complications such as water, electrolyte imbalance, and also lead to hyponatremia and hypokalemia, which at the same time is also a major cause of secondary intracranial cerebral edema.
Considering the disadvantages of standard decompressive craniectomy, we designed this new decompressive craniectomy to make up for the disadvantages of standard decompressive craniectomy to some extent. Its advantages include: 1) full exposure of the lobe, the temporal lobe, and the parietal lobe, making the decompression more complete; removing about 95% of the unilateral supratentorial acute intracranial hematoma, and removing more effectively the hematoma in the anterior skull base and the middle concave bottom, which is conducive to the self-healing of hernia. Full exposure of the skull base, and the anterior longitudinal can clear skull base cistern hemorrhage more precisely, which is conducive to smooth cerebrospinal fluid circulation [14]; 2) full exposure of the veins on the surface and in the depth of the brain such as veins of the Labbe and the lateral fissure veins. Meanwhile the lateral fissure cistern and the skull base cistern are fully opened in surgery. Removing the inferior vena hemorrhage, releasing the bloody cerebrospinal fluid, and relieving vasospasm will smoothen venous return, relieve cerebral edema and infarction, while reducing the incidence of arachnoid adhesion and arachnoid particles clogging, and reconstructing the cerebrospinal fluid circulation [14,15]; 3)Bleeding of the superior sagittal sinus, the bridging veins, the transverse sinus will be controlled, as well as bleeding of the anterior cranial fossa, the middle cranial fossa, and the skull base, so that posttraumatic bleeding will be stopped more thoroughly, and the chance of a second surgery and the incidence of postoperative complications after the second surgery will be reduced; 4)The range of the bone window is large enough so the brain is fully exposed, reducing cerebral contusion and laceration and postoperative complications such as delayed intracerebral hematoma caused by excessive or inappropriate traction of the brain tissue, and reducing the incidence of malignant intracranial hypertension [2,16,17]; 5) a more thorough reparation of the torn dura, reducing the incidence of cerebrospinal fluid leakage.
We found through comparison that the incidences of postoperative subdural effusion and hydrocephalus of the new decompressive craniectomy were obviously smaller than standard decompressive craniectomy. We analyzed the reason of the decrease of subdural effusion: if the intracranial pressure of patients has not been improved, a large dose of the dehydration drugs and dehydrants will be used in postoperative treatment, thereby increasing the emergence of subdural effusion and the effusion quantity and bringing unnecessary damage and economic burden for the prognosis of patients. However, the intracranial pressure relieved significantly after the improved decompressive craniectomy, reducing the application and action time of dehydration drugs. The subarachnoid cerebrospinal fluid circulation is smoother than after the standard decompressive craniectomy, reducing the risk of subdural effusion.
According to the literature, usually in patients with severe traumatic brain injury, the incidence of hydrocephalus is about 10%, yet among our patients in the test group, there were only three cases of postoperative hydrocephalus, significantly lower than that reported in the literature. We analyzed the possible reasons: 1) since the range of the removed bone flap in the standard decompressive craniectomy is very small, inadequate decompression and other reasons after decompressive craniectomy will lead to postoperative brain tissue bulging and displacement. The basal cistern compression has not been significantly alleviated, blocking cerebrospinal fluid circulation; 2) The lateral fissure cistern and the skull base cistern have not been opened during the operation, failing to release the bloody and (or) inflammatory cerebrospinal fluid effectively, causing arachnoid membrane adhesion and disorder of cerebrospinal fluid circulation and malabsorption, leading to postoperative hydrocephalus, bringing unnecessary damage and economic burden for the prognosis of patients; yet the improved decompressive craniectomy decompress thoroughly, the basal cistern compression is significantly eased, meanwhile the lateral fissure cistern and the skull base cistern are opened. The bloody cerebrospinal fluid is released, smoothening the cerebrospinal fluid circulation and reducing the incidence of subarachnoid adhesion, thereby reducing the incidence of hydrocephalus and improving prognosis for patients [2,8,18].
Disadvantages of the new decompressive craniectomy
The incidence of poor healing of scalp incisions and postoperative intracranial infection of the new decompressive craniectomy are higher than the standard decompressive craniectomy. In the test group, 3 patients had poor scalp healing, and 5 patients had intracranial infection.
By our analysis the possible causes of poor scalp healing are: 1) surgical incision: the scalp is rich in blood supply, mainly from the orbitofrontal artery, the superficial temporal artery and various branches, yet due to the large surgical incision on the scalp, damage to the branches of the superficial temporal artery is inevitable, causing poor or prolonged wound healing on the scalp; 2) Patients of severe brain injury need comprehensive treatment combined with mild hypothermia, during the process of which the head wears an ice cap for cooling. The low temperature reduces the blood circulation of the scalp, increasing the incidence of poor wound healing [13].
By our analysis the causes of increased postoperative infection may be that:severe brain injuries are often accompanied by varying degrees of coma and vomiting, affecting the normal physiological function of patients, weakening immunity, and increasing infection rates. The liquefaction and necrosis of oedematous brain tissues offer a very good medium for bacteria. Infections are easy to occur due to intracranial and extracranial communication in open craniocerebral injuries. Related research has found that the number of operations, the operative time, and whether hypoproteinemia and cerebrospinal fluid leakage have occurred are high risk factors of intracranial infection [19,20]. The high risk of intracranial infection in the new decompressive craniectomy is due to: 1) big surgical wound, poor local blood supply, and the incision which is close to the bacteria area through the parietal tuber; 2) long surgical incisions, large amounts of incision sutures, the large area of artificial dura mater required in intraoperative dura mater reparation, as well as the increases of the number and area of foreign matter, increasing the rate of infection; 3) possible open scalp lacerations and open fractures in surgical areas, which needs debridement first, and repeated rinsing by disinfectants such as saline, iodine and 2% of hydrogen peroxide, leading to wet draping, and increasing the chances of infection. In order to reduce postoperative scalp necrosis and the incidence of intracranial infection, surgical incisions and the drainage tube apparatus should be observed closely after the surgery. Dressings and drainage bags should be replaced regularly. The ward should be ventilated and disinfected on time to maintain environmental hygiene. And medical care should be strengthened [2,21].
Conclusion
The new decompressive craniectomy has improved the prognosis, laid a good foundation for comprehensive postoperative treatment, and reduced disability and mortality rates in the treatment of patients with severe and extra severe craniocerebral injury. It is rather significant in clinical promotion and practice.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 81472343).
Disclosure of conflict of interest: none.
References
- Baiyun L. Application of minimally invasive surgery in traumatic brain injury. Chin J Traumatol. 2014;17(6):313-6.
- Timofeev I, Hutchinson PJ. Outcome after surgical decompression of severe traumatic brain injury. Injury. 2006;37(12):1125-32.
- Fujita Y, Algarra NN, Vavilala MS, Prathep S, Prapruettham S, et al. Intraoperative secondary insults during extracranial surgery in children with traumatic brain injury. Childs Nerv Syst. 2014;30(7):1201-8.
- Qiao Y, Feng H, Zhao T, Yan H, Zhang H, et al. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;15(1):154.
- von der Brelie C, Stojanovski I, Meier U, Lemcke J. Open Traumatic Brain Injury Is a Strong Predictor for Aseptic Bone Necrosis after Cranioplasty Surgery: A Retrospective Analysis of 219 Patients. J Neurol Surg A Cent Eur Neurosurg. 2016;77(1):019-24.
- Quintard H, Lebourdon X, Staccini P, Ichai C. Decompression surgery for severe traumatic brain injury (TBI): A long-term, single-centre experience. Anaesthesia Critical Care & Pain Medicine. 2015; 34(2):79-82.
- Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90(2):187-96.
- Tagliaferri F, Zani G, Iaccarino C, Ferro S, Ridolfi L, Basaglia N, Hutchinson P, Servadei F. Decompressive craniectomies, facts and fiction: a retrospective analysis of 526 cases. Acta neurochirurgica. 2012;154(5):919-26.
- Kim YJ. The impact of time to surgery on outcomes in patients with traumatic brain injury: a literature review. International Emergency Nursing. 2014;22(4):214-9.
- Shutter LA, Timmons SD. Intracranial pressure rescued by decompressive surgery after traumatic brain injury. N Engl J Med 2016; 375:1183-4.
- Lombardo S, Scalea T, Sperry J, Coimbra R, Vercruysse G, et al. Neuro, trauma, or med/surg intensive care unit: Does it matter where multiple injuries patients with traumatic brain injury are admitted? Secondary analysis of the American Association for the Surgery of Trauma Multi-Institutional Trials Committee decompressive craniectomy study. Journal of Trauma and Acute Care Surgery. 2017;82(3):489-96.
- Mrozek S, Lonjaret L, Jaffre A, Januel AC, Raposo N, et al. Reversible Cerebral Vasoconstriction Syndrome with Intracranial Hypertension: Should Decompressive Craniectomy Be Considered. Case reports in neurology. 2017;9(1):6-11.
- Brown DA, Wijdicks EF. Decompressive craniectomy in acute brain injury. Handbook of clinical neurology. 2017;140:299.
- Van der Werf AJ. Surgical management of acute subdural hematomas. Clin Neurol Neurosurg. 1975;78(3):161-70.
- Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, et al. Surgical management of acute subdural hematomas. J Neurosurg. 2006;58(3):S2-16.
- Huang CT, Lin WC, Ho CH, Tung LC, Chu CC, et al. Incidence of severe dysphagia after brain surgery in pediatric traumatic brain injury: a nationwide population-based retrospective study. J Head Trauma Rehabil. 2014;29(6):E31-6.
- Honeybul S, Ho KM, Lind CR, Gillett GR. The current role of decompressive craniectomy for severe traumatic brain injury. J Clin Neurosci. 2017; 43:11-15.
- Hayman EG, Kurland DB, Grunwald Z, Urday S, Sheth KN, et al. Decompressive Craniectomy in Neurocritical Care. InSeminars in neurology. 2016;36:(6)508-519.
- Lo YT, See AA, King NK. Decompressive Craniectomy in Spontaneous Intracerebral Hemorrhage: A Case-Control Study. World Neurosurgery. 2017;103: 815-820.
- Tang C, Bao Y, Qi M, Zhou L, Liu F, et al. Mild induced hypothermia for patients with severe traumatic brain injury after decompressive craniectomy. J Crit Care. 2017;39:267-70.
- Lammy S, Fivey P, Sangra M. Decompressive craniectomy for malignant middle cerebral artery infarction in a 16-year old boy: a case report. J Med Case Rep. 2016;10(1):368.