Research Article - Biomedical Research (2017) Volume 28, Issue 5
Analysis of pediatric empyema treated with surgery
Muharrem Cakmak* and Atilla DurkanThoracic Surgery Clinic, Gazi Yasargil Education and Research Hospital, Diyarbakir, Turkey
- *Corresponding Author:
- Muharrem Cakmak
Gazi Yasargil Education and Research Hospital
Thoracic Surgery Clinic, Diyarbakir, Turkey
Accepted date: October 15, 2016
Abstract
Introduction: Pediatric empyema is the most common complication of childhood pneumonia. Our aim is to share the characteristics and treatment methods of pediatric empyema treated with surgery.
Materials and Methods: 66 pediatric patients with pleural empyema were included in the study. Patients were classified as patients treated with tube thoracostomy and thoracotomy. The characteristics of the groups were investigated. The factors that are effective in the application of tube thoracostomy and thoracotomy were evaluated. P<0.05 was considered significant.
Results: The mean age was 5.36 ± 3.85. While 38 (%58) of the patients were male, 28 (42%) were female. 52 (79%) of patients were underwent tube thoracostomy, while 14 (%21) of patients were performed thoracotomy. The average age, the presence of loculations, purulent fluid aspiration with thoracentesis, the average pH, the average glukoz and the average LDH were found to have a significant effect on the application of thoracotomy (p<0.05).
Conclusion: Pediatric empyema requires a multidisciplinary approach. Early diagnosis and treatment are essential. Tube thoracostomy is the primary method of treatment. However, decortication with thoracotomy should be the primary method in the case of loculations and purulent fluid aspiration with thoracentesis.
Keywords
Pediatric empyema, Tube thoracostomy, Thoracotomy.
Introduction
Pediatric empyema is the most common complication of childhood pneumonia [1]. Effusion develops in 40-50% of the children having pneumonia. 10% of them are complicated and require drainage [2]. The possible causes of empyema formation in these patients are not exactly known. The major risk factors which influence the development of the disease can be defined as; being under the age of 3, having chickenpox, having long-lasting fever (>7 days), having received antibiotic treatment, and developing accompanying abdominal pathologies [3]. Empyema should be investigated in children diagnosed with pneumonia and having no clinical improvement within 48 hours despite treatment with antibiotics [4].
Empyema occurs in three stages as exudative, fibrinopurulent and organization stage. Treatment depends on the stage of the disease. Early diagnosis allows conducting the appropriate antibiotic therapy and surgery [5]. While antibiotic treatment and drainage of liquid through aspiration are adequate during the early stages, tube thoracostomy, fibrinolytictherapy with tube thoracostomy, video-assisted thoracoscopic surgery (VATS), or thoracotomy are performed at the later stages [6]. Mortality is around 0-10.8% in childhood empyema [7]. Mortality rate increases as the age decreases.
In this study, our aim is to share the characteristics and treatment methods of pediatric empyema treated with surgery.
Material and Methods
Patients
66 patients who were between the ages of 1-16, hospitalized due to pleural empyema and treated with surgical methods between January 2004 and December 2015 were included in the study.
Procedures
The files of the patients were analyzed retrospectively. Age, gender, symptoms, location of disease, the methods for diagnosis, clinical findings, laboratory (pH, Lactic dehydrogenase (LDH), Glucose) and microbiology (gram stain, culture) results were evaluated. Surgical methods and results, duration of hospitalization, and mortality and morbidity were investigated. Patients were classified as patients treated with tube thoracostomy or thoracotomy. The characteristics of the groups were investigated. The factors effective in the application of tube thoracostomy and thoracotomy were evaluated.
Inclusion and exclusion criteria
Patients treated with surgical methods were included in the study. The methods of treatment which received positive results were considered. The patients having positive results with fibrinolytics were included in tube thoracostomy group.
Statistical analysis
Data were analyzed by Statistical Package for the Social Sciences (SPSS 21, Chicago, IL, USA). Numerical data were expressed as mean ± standard deviation and qualitative data as percent. Mann-Whitney U test, Chi-Square test, Fisher Exact test were used in study between double and triple groups. The lowest level of significance was considered as p<0.05. The averages of countable parameters were determined. Chi-Square test (cross-tab) and fisher exact test were used to learn the percentage of uncountable parameters and to determine significant differences.
Findings
The mean age of the patients was 5.36 ± 3.85. 38 (58%) of the patients were male, while 28 (42%) were female. Disease was on the right in 36 (55%) patients, whereas it was on the left in 30 (45%). All of the patients who were referred to us by the clinics of infectious disease or pediatrics, and failed to respond to the medical treatment were consulted. All effusions were exudative. The mean pleural fluid was pH: 6.73 ± 0.31, the mean LDH was 759.01 ± 233.64, and glucose was 38.19 ± 6.43. 6 (9%) patients had positive gram stain and culture, while 60 (91%) had negative. Loculations were present in 17 (26%) patients. Moreover in 49 (74%) patients, fluid which had purulent characteristic was aspirated. In the treatment, 52 of patients (79%) were underwent tube thoracostomy, while 14 (21%) of patients were performed thoracotomy (Table 1).
| Variables | Number / Percentage | Results |
|---|---|---|
| Male/Female | N (%) | 38/28 (57.6%/42.4%) |
| Age | (Mean ± SD) | 5.36 ± 3.8526 |
| PH | (Mean ± SD) | 6.73 ± 0.3101 |
| Lactate dehydrogenase | (Mean ± SD) | 759.01 ± 233.6453 |
| Glucose | (Mean ± SD) | 38.19 ± 6.4336 |
| Right pleural effusion | n (%) | 36 (54.5%) |
| Left pleural effusion | n (%) | 30 (45.5%) |
| Gram stain/culture positive | n (%) | 6 (9.1%) |
| Gram stain/culture negative | n (%) | 60 (90.9%) |
| Loculation positive | n (%) | 17 (25.8%) |
| Loculation negative | n (%) | 49 (74.2%) |
| Purulent fluid positive | n (%) | 10 (15.2%) |
| Purulent fluid negative | n (%) | 56 (84.8%) |
| Tube Thoracostomy | n (%) | 52 (78.8%) |
| Thoracotomy | n (%) | 14 (21.2%) |
| n: Number, SD: Standard Deviation | ||
Table 1. Demographic distribution of patients
The mean age of the patients treated with tube thoracostomy (n: 52) was 4.75 ± 3.42. While 30 (58%) of the patients were male, 22 (42%) were female. The disease was on the right in 30 (58%) patients, whereas it was on the left in 22 (42%). The mean pleural fluid was pH: 6.85 ± 0.21, LDH was 659.13 ± 141.82, and glucose was 40.25 ± 5.68. While 3 (6%) patients had positive gram stain and cultures, 49 (%94) had negative gram stain and cultures. 3 (6%) patients had loculations (Table 2).
| Tube Thoracostomy | Thoracotomy | P value | |
|---|---|---|---|
| Male N (%) Female N (%) |
30 (57.7%) 22 (42.3%) |
8 (57.1%) 6 (42.9%) |
1.000** |
| The average age (mean±SD) | 4.76 ± 3.43 | 7.61 ± 4.61 | <0.026* |
| Right side of the chest N (%) Left side of the chest N (%) |
30 (57.7%) 22 (42.3%) |
6 (42.9%) 8 (57.1%) |
χ2: 0.979*** P = 0.322 |
| Gram stain/culture positive N (%) Gram stain/culture negative N (%) |
3 (5.8%) 49 (94.2%) |
3 (21.4%) 11 (78.6%) |
0.104** |
| Loculation positive N (%) Loculation negative N (%) |
3 (5.8%) 49 (94.2%) |
14 (100%) - |
<0.0001** |
| Purulent fluid positive N (%) Purulent fluid negative N (%) |
- 52 (100%) |
10 (71.4%) 4 (28.6%) |
<0.0001** |
| pH mean ± SD | 6.86 ± 0.215 | 6.30 ± 0.186 | <0.0001* |
| LDH mean ± SD | 659.13 ± 141.82 | 1130.00 ± 76.96 | <0.0001* |
| Glukoz mean ± SD | 40.25 ± 5.681 | 30.57 ± 0.938 | <0.0001* |
| LDH: Lactate dehydrogenase, P*: Mann-witney U test, P**: Fisher's exact test, P***: Chi-Square test | |||
Table 2. Comparison of the treatment of the patient.
The mean age of patients treated with thoracotomy (n: 14) was 7.60 ± 4.60. While 8 patients (57%) were male, 6 (33%) of patients were female. Disease was on the right in 6 (33%) patients, while it was on the left in 8 (57%). The mean pleural fluid was pH 6.29 ± 0.18, LDH was 1130 ± 76.96, and glucose was 30.57 ± 0.93. In three (21%) patients, gram stain and cultures were positive while in 11 cases (79%), they were negative. 14 (100%) of the patients had loculations. During the examination through Thoracentesis, it was observed that fluid having purulent characteristics was aspirated in 10 (71%) patients (Table 2).
The average age (p:0.026), the presence of loculations (p: 0.0001), purulent fluid aspiration with thoracentesis (p:0.0001), the average pH (p:0.0001), the average glukoz (p: 0.0001) and the average LDH (p: 0.0001) were found to have a significant effect on the application of thoracotomy (Table 2).
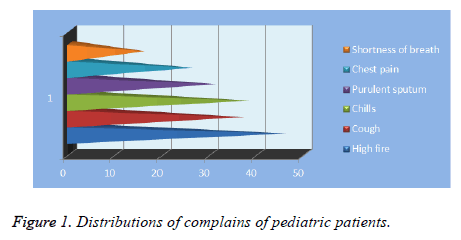
The main complaints were fever (n: 44, 67%), cough (n: 37, 56%), chills (n: 38, 56%), purulent sputum (n: 31, 46%), chest pain (n: 26, 38%), and dyspnea (n: 16, 24%) (Figure 1). The most frequently used imaging method was chest radiography, the second was computed tomography of thorax and the least frequent one was thoracic ultrasonography.
The microorganisms detected in patients were S. aureus in 3 patients, S. pneumoniae in 2 cases, group A Streptococcus in 1 patient, and E. coli in 1 case.
All patients got intravenous antibiotic therapy in accordance with the opinions of the infectious disease and pediatric clinics. Two (3%) patients died due to sepsis. Postoperative 6 (9%) patients had expansion defects, 2 patients (3%) had incision infection, and 8 (12%) developed atelectasis. The patients' average length of stay was 8 ± 3.4 days.
Conclusion
Pleural effusion is a common finding of pleural diseases. Pleural effusion and empyema often occur as a complication of bacterial pneumonia. Empyema development increases morbidity and mortality by exacerbating the prognosis of pneumonia [8]. Even empyema can be seen at any age, it is more common, especially under 2 years of age [2]. Therefore, in this age group, patients given oral antibiotics for the treatment of pneumonia should be closely monitored, and the findings such as fever, dyspnea, and orthopnoea should be noted. In addition, long term antibiotics use has been reported as a risk factor for the development of the parapneumonic empyema [3]. All of our patients were given medical treatment with the diagnosis of pneumonia.
Although seen in both hemithorax, pleural effusion is more visible on the right side [1,2,9]. Caksen et al. [10] reported that the disease was on the right in 39.7% of cases, on the left in 32.3%, and bilateral in 27.9%. In our study, right hemithorax was present in 55% of the patients.
The most common symptoms of patients are cough, tachypnea, fever, chest pain, abdominal pain and vomiting [2]. Diagnosis was given through clinical findings, laboratory tests and imaging. Increase in leukocytes and c-reactive protein values are typical symptoms [3,11]. The most common symptoms of our patients were fever (n: 44, 67%), cough (n: 37, 56%), chills (n: 38, 56%), and purulent sputum (n: 31, 46%).
Thoracentesis is a simple and secure method for pleural fluid analysis. Metabolism of bacteria and leukocytes in the pleural fluid decrease pH and glucose levels of pleural fluid. Lactate dehydrogenase (LDH) increases as a result of the lysis of polymorpho nuclear leukocytes and other phagocytes in pleural fluid. The distinction of transudate and exudate is made in accordance with the criteria developed by Light et al. [12]. In the presence of only one of the following parameters; pleural fluid glucose <50 mg/dL, pleural fluid / serum LDH>0.6 and pleural fluid/serum protein >0.5, the diagnosis of exudate is given. Empyemas are fluids having exudate properties. All of our patients were characterized exudate.
The most frequently used imaging method is the chest x-ray. Ultrasonography is an appropriate method in detecting and identifying septations, loculations, and localization during thoracentesis. Tomography of thorax is the most appropriate method for the evaluation of parenchymal disease [13].
Parapneumonic effusions are often a complication of pneumonia caused by S. pneumoniae, H. influenzae, S. aureus and group A Streptococcus [14]. S. aureus is a pathogenic frequently isolated in children and adults. However, in some studies, S. pneumoniae has been reported to be more common factors [3]. In this study, the most frequently isolated microorganisms were S. aureus (n: 3, 4.4%), S. pneumoniae (n: 2, %2.9), group A Streptococcus (n: 1, %1.4), and E. coli (n: 1, %1.4).
There are three stages of empyema. Exudative stage (stage 1) is an empyema stage which responds effectively to thoracentesis or chest tube drainage. In fibrinopurulent stage (stage 2), previously sterile pleural effusion becomes infected with the accumulation of polymorphonuclear leukocytes and debris. Viscous liquid and fibrin deposition may cause loculations. At this stage, it is difficult to drain the liquid, but the majority of cases respond chest tube drainage. Transition from stage 1 to stage 2 is rapid. This transition occurs within 24-48 hours. In the organization stage (stage 3), it has now become chronic. A thick inelastic pleural peel covering and pressuring lung is present [6,14,15].
In treatment, the stage of empyema is very important. All cases should be given to intravenous antibiotics, and those antibiotics should be selected for the most common pathogens of pneumonia in patients' age group. Whether the infection is society or hospital-acquired should be considered [14]. The continuation of fever after 48 hours from the beginning of antibiotic therapy requires the drainage of pleural effusion [12,16].
Chest tube drainage is the first treatment approach in the drainage of the parapneumonic effusion. If pleural fluid is pH <7 and / or glucose <40 mg/dL and/ or in the presence of positive gram stain or culture, drainage is essential [16]. Caksen et al. [10] reported that they treated 60.2% of patients with empyema through chest tube drainage, and Chen et al. [6] reported that they treated 87% of patients with chest tube drainage. Patients who do not respond chest tube drainage should be assessed in terms of blockage in the tube, loculations, thick pleural surfaces, and bronchopleural fistula [14].
Patients with fibrinopurulent and organized empyema may not respond to the pleural drainage. In this case, the combination of loculations, thoracotomy aiming the removal of the fibrous tissue surrounding the lung (decortication) can be performed [14].
In a retrospective study conducted by Thourani et al. [17], decortication was reported to be more effective than tube thoracostomy. Additionally, delayed surgical drainage causes higher morbidity and mortality and extends the length of stay in patients with pleural empyema [18]. In some cases, pleural thickening after surgery is ongoing, but it is significantly improved within 3 months in most cases [14]. However, this improvement may be in 5-6 months [6].
In our study, 52 of patients (79%) were underwent tube thoracostomy, while 14 (21%) of patients were performed thoracotomy. In addition, the average age (p: 0.026), the presence of loculations (p: 0.0001), purulent fluid aspiration with thoracentesis (p: 0.0001), the average pH (p: 0.0001), the average glukoz (p: 0.0001) and the average LDH (p: 0.0001) were found to have a significant effect on the application of thoracotomy (Table 2).
The mortality rate in children with empyema has been reported to be 0-10.8%. Mortality is much higher in children under 1 year of age [7,8]. In our study, the mortality rate was 3%.
As a result, childhood empyema requires a multidisciplinary approach. Early diagnosis and treatment are essential. Close monitoring of sick children and the evaluation of complex, conflicting clinical, radiological, biochemical and microbiological data are essential. Tube thoracostomy is the primary method of treatment. However, in the case of the presence of loculations, and purulent fluid aspiration with thoracentesis, decortication with thoracotomy should be the primary method of treatment to prevent the loss time, and avoid increasing mortality and morbidity.
References
- Brandenburg JA, Marrie TJ, Coley CM, Singer DE, Obrosky DS. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med 2000; 15: 638-646.
- Wheeler JG, Jacobs RF. Pleural effusions and empyema. In: Feigin RD, Cherry JD, Demmler-Harrison GJ, Kaplan SL. Text book of Pediatric Infectious Diseases, 6th ed. Philadelphia: Saunders Elsevier 2009: 325-335.
- Lahti E, Peltola V, Virkki R, Alanen M, Ruuskanen O. Development of parapneumonic empyema in children. Acta Paediatr 2007; 96: 1686-1692.
- Balfour-Lynn IM, Abrahamson E, Cohen G, Hartley J, King S. BTS guidelines for the management of pleural infection in children. Thorax 2005; 60 Suppl 1: i1-21.
- Proesmans M, De Boeck K. Clinical practice: treatment of childhood empyema. Eur J Pediatr 2009; 168: 639-645.
- Chan PW, Crawford O, Wallis C, Dinwiddie R. Treatment of pleural empyema. J Paediatr Child Health 2000; 36: 375-377.
- Hoff SJ, Neblett WW, Edwards KM. Parapneumonic empyema in children: Decortication hastens recovery in patients with severe pleural infections. Pediatr Infect Dis J 1990; 10: 194-199.
- Davies CW, Gleeson FV, Davies RJ; Pleural Diseases Group, Standards of Care Committee, British Thoracic Society. BTS guidelines for the management of pleural infection. Thorax 2003; 58 Suppl 2: ii18-28.
- Dass R, Deka NM, Barman H, Duwarah SG, Khyriem AB. Empyema thoracis: analysis of 150 cases from a tertiary care centre in North East India. Indian J Pediatr 2011; 78: 1371-1377.
- Caksen H, Oztürk MK, Yüksel S, Uzüm K, Ustünbaş HB. Parapneumonic pleural effusion and empyema in childhood. J Emerg Med 2003; 24: 474-476.
- Lin CJ, Chen PY, Huang FL, Lee T, Chi CS, Lin CY. Radiographic, clinical, and prognostic features of complicated and uncomplicated pneumonia in children. J Microbiol Immunol Infect 2006; 39: 489 - 95.
- Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507-513.
- Kearney SE, Davies CW, Davies RJ, Gleeson FV. Computed tomography and ultrasound in parapneumonic effusions and empyema. Clin Radiol 2000; 55: 542-547.
- Mocelin HT, Fischer GB. Epidemiology, presentation and treatment of pleural effusion. Paediatr Respir Rev 2002; 3: 292-297.
- de Souza A, Offner PJ, Moore EE, Biffl WL, Haenel JB. Optimal management of complicated empyema. Am J Surg 2000; 180: 507-511.
- Islam S, Calkins CM, Goldin AB, Chen C, Downard CD. The diagnosis and management of empyema in children: a comprehensive review from the APSA Outcomes and Clinical Trials Committee. J Pediatr Surg 2012; 47: 2101-2110.
- Thourani VH, Brady KM, Mansour KA, Miller JI Jr, Lee RB. Evaluation of treatment modalities for thoracic empyema: a cost-effectiveness analysis. Ann Thorac Surg 1998; 66: 1121-1127.
- Rizalar R, Somuncu S, Saraç A, Bernay F, Gürses N. Postpnömonik ampiyemde erken dekortikasyon. Pediatrik Cerrahi Dergisi 1993; 7: 6-9.