Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2024) Volume 14, Issue 104
ANALYSIS OF ALLELIC VARIANTS OF CYP2C19 IN GASTRIC BIOPSY SAMPLES
Sergio David Angulo1*, David Cano1, César Augusto Cortés1, Nicolás Jiménez1, Álvaro Javier Rodríguez1, Luis Fernando Pérez1, Paula Tatiana Uribe1, Diego Alejandro Ruiz1, Echeverry Jhon Fredy Betancur Perez2
1Department of Medicine, University of Manizales, Colombia
2Department of molecular biology, University of Manizales, Colombia
- *Corresponding Author:
- Sergio David Angulo
Department of Medicine
University of Manizales, Colombia
E-mail: sdangulo8128@umanizales.edu.co
Received: 08-Mar-2024, Manuscript No. AABPS-24-128725; Editor assigned: 09-Mar-2024, Pre QC No. AABPS-24-128725 (PQ); Reviewed: 23-Mar-2024, QC No. AABPS-24-128725; Revised: 28-Mar-2024, Manuscript No. AABPS-24-128725 (R); Published: 04-April-2024, DOI: 10.35841/aabps-14.104.221
Citation: Ding J. Analysis of allelic variants of cyp2c19 in gastric biopsy samples. Asian J Biomed Pharm Sci.2024; 14(104):221
Abstract
Helicobacter-related chronic gastritis is pathology with a high prevalence, it is considered the most common form of gastritis worldwide. The cornerstone of the therapy for this entity is proton pump inhibitors (PPIs), whose mechanism of action is the decrease in acid secretion. In general, this pharmacological group is characterized by a good profile, with a low frequency of adverse drug reactions. PPIs are metabolized by hepatic enzymes of the CYP2C19 family, which are encoded by a gene with polymorphisms that affect the way in which the drug is metabolized, implications that clinically mean the need for a higher or lower dose of the drug for optimal antisecretory effect and less development of adverse drug reactions. To analyze the allelic variants of CYP2C19 in patients with gastric disorders treated with PPIs. A descriptive, retrospective and observational research will be carried out. The analysis population are DNA samples from gastric biopsies stored in a DNA bank. A descriptive statistical analysis will be carried out through SPSS and the use of proton pump inhibitors and the diagnosis of gastric injury are applied as inclusion criteria. The determination of the alleles of the selected samples will be carried out through PCR amplification, subsequently they are subjected to digestion with restriction enzymes SmaI and BamH. The visualization of the restriction products will be carried out in agarose gels at 2% (w/v) stained with the GelRedTM dye. Contribute to the development of personalized medicine in patients with acid secretion disorders, treated with PPIs, thus improving the efficiency of therapy through the calibration of the dose according to the metabolizer phenotype.
Key words
Proton pump inhibitors, Polymorphisms, CYP2C19, Gastric Injury
Introduction
Chronic gastritis related to Helicobacter is a pathology with a high prevalence in the general population. It is considered the most common form of gastritis worldwide. Medications from the group of gastritis inhibitors are used to treat this pathology. proton pumps (PPIs), which decrease gastric acid production through inhibition of the proton- potassium ATPase pump. In general, this pharmacological group is characterized by a good profile, with a low frequency of adverse drug reactions. PPIs are metabolized by liver enzymes of the CYP2C19 family, which are encoded by a gene with polymorphisms that affect the way in which the medication is digested so that there will be individuals who metabolize the drug more quickly or more slowly, implications which clinically mean the need for a higher or lower dose of the medication for an optimal antisecretory effect and less development of adverse drug reactions [1].
Diseases related to alteration in acid secretion are a broad group of pathologies that include disorders such as chronic gastritis associated with Helicobacter Pylori, Zollinger-Ellison syndrome, gastroesophageal reflux disease (GERD). ) and acid peptic disease (1). Currently, chronic gastritis associated with Helicobacter Pylori is the most common form of chronic gastritis in humans. According to studies in Latin America and the Caribbean, the prevalence of Helicobacter Pylori infection is approximately 57%, with a increase in prevalence along with age [2-4]
Therapy for the management of these disorders is based on the use of proton pump inhibitors (PPIs), which are among the most widely used medications worldwide [5]. Within this group of medications there is omeprazole, which is used for the treatment of GERD, peptic ulcer disease and the eradication of Helicobacter Pylori [6]. Among its adverse drug reactions (ADRs) are hypergastrinemia and increased risk of gastric neoplasia, acute interstitial nephritis, bone fractures, and increased risk of liver disease related to cirrhosis [7].
Proton pump inhibitors are a group of medications, metabolized by the liver enzyme CYP2C19, which is encoded by a polymorphic gene. There are four phenotypes that influence the rate of PPI metabolism: CYP2C19 * 17 / * 17 (ultra- rapid metabolizers), CYP2C19 *1/*17 (extensive metabolizers), CYP2C19 *1/*1 (normal metabolizer) , CYP2C19 *1/*2 and *1/3 (intermediate metabolizers),CYP2C19 *3/*3, *2/*3 and 2*/2* (poor metabolizers) [8]. Currently, in Manizales, no studies have yet been carried out on the allelic variations of CYP2C19 in patients with gastric disorders, however it is valid to carry out a study with the purpose of knowing the allelic frequency of the gene to determine the most frequent phenotype and contrast it with the findings of other pharmacogenomic studies carried out in cities at a national and international level; so that it allows a formulation and dosage of the medication according to the pharmacogenomic characteristics of the population, impacting a personalized medical practice [9].
Literature review
Chronic gastritis has been known and studied since the first decades of the 20th century but did not receive more attention until 1982 after the discovery of Helicobacter pylori by Warren and Marshall. Helicobacter Pylori is the main risk factor for chronic gastritis which, in turn, promotes the development of acid peptic disease, gastric and duodenal ulcer, gastric carcinoma and MALT type lymphoma (mucous associated lymphoid tissue). [10-13]. Gastritis is defined as a pathological process consisting of an acute or chronic inflammation of the gastric mucosa that is produced by various factors, both exogenous and endogenous, and this produces symptoms in relation to the disease and its effects. Presence can be suspected clinically, and endoscopy and histological examination are mandatory for confirmation. However, it is a term that is commonly used incorrectly to refer to any condition associated with the upper gastrointestinal tract. In addition, the term gastropathy must be considered as any pattern of non-specific microscopic lesion with or without the presence of any inflammatory cellular infiltrate. Both gastritis and gastropathy can occur asymptomatically, so this aspect cannot be considered for their diagnosis. To determine endoscopic absence of gastritis, regular gastric mucosa must be evident, pink in color, uniform, without structural alterations, gastric folds no thicker than 5 mm, absence of adherent fibrinopurulent exudate and adequate expansion. of the gastric mucosa when insufflated with air. However, inflammatory changes induce apoptosis and damage of the gland, which can lead to regeneration and change of the gastric parenchyma resulting in fibrosis or metaplasia [14].
To understand the pathophysiological process of gastritis, it is necessary to be clear about the protective and damaging factors of the mucosa since the disease is the result of an imbalance between these. Depending on the degree of imbalance, gastritis of varying intensity will develop. The most important cytotoxic factors are hydrochloric acid, pepsin, medications such as aspirin or non- steroidal anti-inflammatory drugs (NSAIDs), bile acids and finally Helicobacter Pylori, and in contrast the most important cytoprotective factors are the mucus layer. that its function is to protect epithelial cells from contact with hydrochloric acid, pepsin and other enzymes that are involved in the digestion process and also to counteract the increase in acidity, the secretion of bicarbonate is developed in defense of epithelial cells, there is also the production of prostaglandins that directly cause an increase in the production of bicarbonate and mucus and also improves mucosal blood flow [15].
Regarding the classification, it can be divided into acute and chronic gastritis. Acute gastritis is an acute mucosal inflammatory process associated with damage to the gastric mucosa, generally of a transient nature, while chronic gastritis refers to chronic inflammatory changes that can eventually lead to atrophy of the mucosa, Epithelial metaplasia, dysplasia and intestinal type gastric cancer. Gastritis is a dynamic process, which can vary from acute to chronic (active or inactive), present in different stages of recovery or atrophy and can lead to complications. Inflammation can be diffuse or predominantly affect the antrum. or the body. (16) The concept of chronic gastritis has always been a source of controversy. With the purpose of eliminating diagnostic confusion, a classification and grading system (Sydney System) was created in 1990 in Sydney (Australia), which makes endoscopic and histological considerations and recommends that the diagnosis of gastritis is made by an integration of etiological, histological and endoscopic information [16]. It suggests that the same grading categories be used (mild, moderate and severe), both for histology and for endoscopic variables [17].
Later in 1994, another meeting was held in Houston (Texas), in which the terminology of the classification was improved and the importance of distinguishing between atrophic and non-atrophic stomachs was emphasized, considering that the names used for each entity were accepted by pathologists and endoscopies. (twenty) In 2017, the Kyoto classification emerged based on the endoscopic characteristics of gastritis associated with Helicobacter pylori, this is a Gram-negative bacterium with bacillary morphology, microaerophilic and that successfully colonizes the gastric mucosa. human. This pathogen is the main etiological agent of various pathologies of the gastrointestinal tract and infects approximately half of the world's population. Along with this bacterium, patterns associated with a high risk of gastric cancer could be identified. This classification system divides patients into three groups: H. pylori-negative patients (no gastritis), patients with current Helicobacter Pylori infection (active gastritis), and patients previously infected with H. pylori (inactive gastritis). The score of five gastritis parameters (atrophy, intestinal metaplasia, gastric fold hypertrophy, nodularity and diffuse erythema) should provide an estimate of the risk of gastric cancer [18-20]. Currently, the classification is used with the updates that were made in Houston and Kyoto, as a major inclusion of the H. Pylori germ and that this microbe must be taken into account in the treatment and management plan to make good medicinal use in pro of the patient, but, taking into account the other factors that favor the growth of this bacteria [21].
In clinical practice, patients will commonly be asymptomatic, although the most common occurrence is heartburn or pain in the epigastrium, heartburn, upper abdominal pain (which may worsen when eating), bloating, lack of appetite, digestive bleeding, nausea, vomiting, feeling of fullness, among others. [22-23]. Incidence of symptoms related to the presence of gastritis is observed that abdominal pain occupies 45% of the cases, in second place is heartburn with 15% and in third place is It is occupied by the presence of vomiting, abdominal distension, flatulence and nausea each represent 10% of the cases found [24]. Proton pump inhibitors (PPIs) are a group of medications widely used for the management of pathologies related to gastric acidity, such as gastroesophageal reflux disease, acute gastritis and the eradication of Helicobacter Pylori [25-27]. PPIs are characterized by a good tolerance profile and low rate of adverse reactions; however, polymorphisms in the main enzyme that metabolizes them, the cytochrome CYP2C19, can affect the pharmacokinetics and pharmacodynamics of the drug, resulting in variable plasma concentrations that lead to responses. variables in individuals with phenotypes other than the alleles (16).| At a global level, pharmacogenomic studies of allelic variants of CYP2C19 have been carried out in various population groups, which show that a variation in the alleles is expressed as phenotypes with normal, increased or decreased metabolism of PPIs and is clinically evidenced as a greater or lower frequency of adverse drug reactions [28]. Clinically important drug interactions with proton pump inhibitors (PPIs) are rare. However, the metabolism of PPIs through the liver enzyme cytochrome P450 may lead to specific drug interactions in some people. The presence of mutations in the CYP2C19 gene leads to higher plasma concentrations of PPI in homozygous subjects. However, if this metabolic pathway becomes saturated, the isoenzymes could become an important target for interactions with many drugs, including warfarin, diazepam, clopidogrel, and phenytoin [29].
Materials and Methods
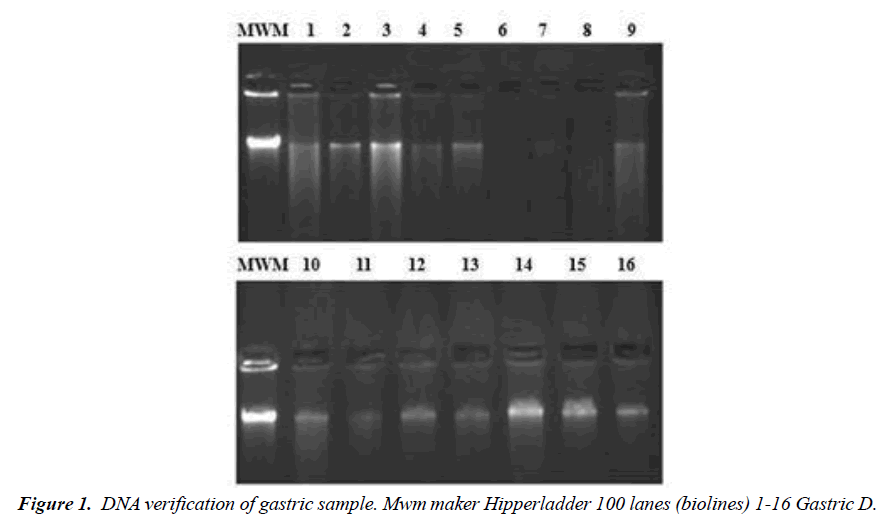
Descriptive, retrospective and observational research was carried out. The analysis population is DNA samples from gastric antrum and body biopsies, stored in a DNA bank at the University of Manizales. The characteristics are histological diagnosis of gastric lesion, both sexes, age over 18 years and residence in the department of Caldas, Colombia. Descriptive statistical analysis is performed and the use of proton pump inhibitors and the diagnosis of gastric injury are applied as inclusion criteria. Verification of DNA quality, for the selection of DNA samples with adequate concentration, is carried out through electrophoresis in agarose gels (Figure 1). The determination of the alleles of the CYP2C19 gene located on the long arm of chromosome 10 was carried out by PCR amplification of the CYP2C19*2 allele, using the primer 5 AATTACAACCAGAGCTTGGC-3[30].
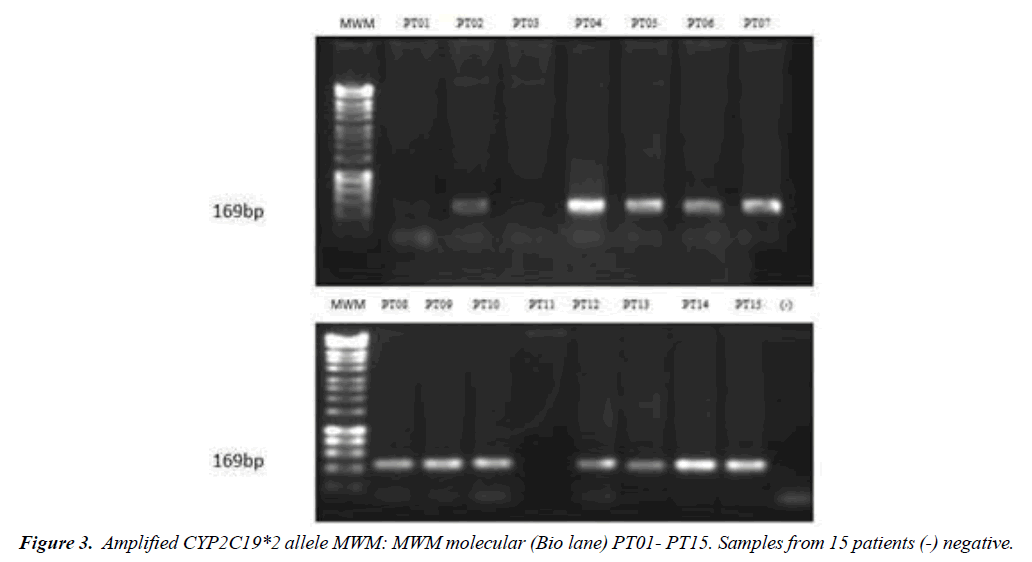
The amplification was carried out in the MultiGeneTM Gradient Thermal Cycler (Labnet), of the molecular biology laboratory of the University of Manizales, under the reaction conditions: denaturation at 95 °C for 5 minutes, followed by 40 cycles of 95°C for 30 seconds, 56-58°C for 15 seconds, 72°C for 30-40 seconds and a final extension at 72°C for 5 minutes . Subsequently, the PCR amplification of the CYP2C19 *2 allele was visualized in 2% (W/V) agarose gels stained with GelRed (28) (Figure 2). The PCR amplification product of the CYP2C19*2 allele with an approximate size of 169 bp was digested with the restriction enzyme SmaI (Jena Bioscience), for which the manufacturer's recommendations were considered. Visualization of the restriction products will be made in 2% (w/v) agarose gels stained with the GelRedTM dye (Figure 3). For statistical analysis, the SPSS® program (IBM, version 24) was used, a value of p < 0.05 is considered statistically significant. The data was analyzed by Fisher's exact test. X2 tests were performed to evaluate the goodness of fit between the observed and expected frequencies (Hardy-Weinberg equilibrium test) [31-34].
Results
CYP2C19*2 obtained an amplification of approximately 169 Pb (Figure 2). The CYP2C19*2 amplifications were digested with the restriction enzyme SmaI. The enzyme cuts generated fragments of 40 bp and 129 bp and a 169 bp fragment - undigested fragment. Homozygous individuals presented two segments of 129 Pb and 40 Pb, in accordance with what was reported by Perez and others. The wild ones present undigested fragments of 169 bp. A frequency of 29% is reported for homozygotes and the remaining 71% corresponds to wild phenotypes (Table 1).
| Wild Type |
Homozygotes |
|---|---|
| 1*/1* | 2*/2* |
| 71 | 0.29 |
Table 1. Genotypes frequency.
Discussion
Cytochromes play an important role in drug metabolism, with CYP2C19 being responsible for approximately 7% of all drugs used in clinical practice and the main person responsible for the metabolism of PPIs (30). CYP2C19 is an enzyme encoded by a polymorphic gene, with its *2 allele being common in the Caucasian population and *3 in the Asian population. The presence of the alleles determines the PPI metabolism phenotypes that clinically imply better Helicobacter Pylori eradication rates and lower risk for acid peptic disease symptoms according to real-life retrospective cohort studies. In Latin America and Colombia Few studies have been conducted on the prevalence of CYP2C19 alleles. In our work we found a higher prevalence than that reported by Arévalo-Galvis and Isaza-Henao. These findings make it possible to compare results and increase genetic information about patients, in order to promote the development of personalized medicine in this region of the world.
References
- Franceschi M, Di Mario F, Leandro G, et al. Acid related disorders in the elderly. Best Practice Res Clin Gastroenterolo. 2009;23(6):839-48.
- Perez?Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2004;9:1-6.
- Banatvala N, Mayo K, Megraud F, et al. The cohort effect and Helicobacter pylori. Journal of infectious diseases. 1993;168(1):219-21.
- Curado MP, de Oliveira MM, de Araújo Fagundes M. Prevalence of Helicobacter pylori infection in Latin America and the Caribbean populations: A systematic review and meta-analysis. Canc Epidemiolo. 2019;60:141-8.
- Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterolo Repor. 2008;10(6):528-34.
- Freedberg DE, Kim LS, Yang Y. Adverse effects of proton pump inhibitors: evidence and plausibility. Internati J Molecu Sci. 2019;20(20):1-5.
- El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opinion Drug Metabol Toxicolo. 2018;14(4):447-60.
- Arevalo-Galvis A, Otero-Regino WA, Ovalle-Celis GN, et al. Prevalence of CYP2C19 polymorphism in Bogotá, Colombia: The first report of allele* 17. Plos one. 2021;16(1):e0245401.
- Gravina AG, Zagari RM, De Musis C, et al. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterolo. 2018;24(29):3204.
- Lehours P, Megraud F. Helicobacter pylori infection and gastric MALT lymphoma. Roczniki Akademii Medycznej w Bialymstoku (1995). 2005;50:54-61.
- Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: Treatment of Helicobacter pylori infection. Official J Americ Colle Gastroenterolo. 2017;112(2):212-39.
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Offic J Ameri Colle Gastroenterolo. 2013;108(3):308-28.
- Zhang HJ, Zhang XH, Liu J, et al. Effects of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of proton pump inhibitors. Pharmacolo Res. 2020;152:104606.
- Bishop JR, Najjar F, Rubin LH, et al. Escitalopram pharmacogenetics: CYP2C19 relationships with dosing and clinical outcomes in autism spectrum disorder. Pharmacogen Genomi. 2015;25(11):548-54.
- Redal MA, Scibona P, Garfi LG, et al. Farmacogenómica de las enfermedades gastroenterológicas. Acta Gastroenteroló Latinoamer. 2012;42(1):64-72.
- Marshall B, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. The lancet. 1984;323(8390):1311-5.
- Dias C. Gastritis Con cuál clasificación endoscópica nos quedamos?. Revista GEN. 2019;73(3):106-11.
- Dixon MF, Genta RM, Yardley JH, et al. Histological classif ication of gastritis and helicobacter pylori infection: An agreement at last? Helicobacter. 1997;2:17-24.
- Villagran CA, Avellaneda LV, López JR, et al. Factores epidemiológicos asociados a la gastritis aguda por Helicobacter pylori en pacientes atendidos en un servicio de gastroenterología. Revis Científic Investigaci Conocimi. 2018;2(3):694-704.
- Martín-Echevarría E, Pereira Juliá A, Torralba M, et al. Evaluación del uso de los inhibidores de la bomba de protones en un servicio de medicina interna. Revis Espa Enfermeda Digesti. 2008;100(2):76-81.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterolo. 2001;121(5):1095-100.
- Liu KH, Kim MJ, Shon JH, et al. Stereoselective inhibition of cytochrome P450 forms by lansoprazole and omeprazole in vitro. Xenobiotica. 2005;35(1):27-38.
- Tang HL, Li Y, Hu YF, et al. Effects of CYP2C19 loss-of-function variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor-based triple therapy regimens: a meta-analysis of randomized clinical trials. PloS one. 2013;8(4):e62162.
- Isaza C, Henao J, Martínez JH, et al. Phenotype-genotype analysis of CYP2C19 in Colombian mestizo individuals. BMC Clini Pharmacolo. 2007;7:1-5.
- Adithan C, Gerard N, Vasu S, et al. Allele and genotype frequency of CYP2C19 in a Tamilian population. Briti J Clini Pharmacolo. 2003;56(3):331-3.
- Vu NP, Nguyen HT, Tran NT, et al. CYP2C19 genetic polymorphism in the Vietnamese population. Annals of Human Biology. 2019;46(6):491-7.
- Echeverry PT, Cañón JC, Clavijo NJ, et al. Uso alternativo del colorante gelred en la tinción de ácidos nucleicos. Archivos Medici. 2013;13(2):160-6.
- García Franco L, JA BF, Muñoz B, et al. Preventive activities in women's care. Prima Attent. 2020;52:125-48.
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacolo Therapeuti. 2013;138(1):103-41.
- Lin YA, Wang H, Gu ZJ, et al. Effect of CYP2C19 gene polymorphisms on proton pump inhibitor, amoxicillin, and levofloxacin triple therapy for eradication of Helicobacter pylori. Medic Scien Monit Internatio Medica J Experime Clini Res. 2017;23:2701.
- Saito Y, Serizawa H, Kato Y, et al. First-line eradication for Helicobacter pylori-positive gastritis by esomeprazole-based triple therapy is influenced by CYP2C19 genotype. World J Gastroenterolog. 2015;21(48):13548.
- Gronich N, Lavi I, Lejbkowicz F, et al. Association of CYP2C19 polymorphism with proton pump inhibitors effectiveness and with fractures in real?life: Retrospective cohort study. Clini Pharmacolo Therapeu. 2022;111(5):1084-92.
- Tang Hui Lin TH, Li Yan LY, Hu Yong Fang HY, et al. Effects of CYP2C19 loss-of-function variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor-based triple therapy regimens: a meta-analysis of randomized clinical trials.
- Isaza C, Henao J, Martínez JH, et al. Phenotype-genotype analysis of CYP2C19 in Colombian mestizo individuals. BMC Clini Pharmacolo. 2007;7:1-5.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref