- Biomedical Research (2013) Volume 24, Issue 2
An update on diagnostic value of biotinidase: From liver damage to cancer: Minireview.
Minnie Faith and Premila Abraham*Department of Biochemistry, Department of Biochemistry, Christian Medical College, Bagayam, Vellore, Tamil Nadu, India
- *Corresponding Author:
- Premila Abraham
Department of Biochemistry
Christian Medical College
Bagayam, Vellore 632002
Tamil Nadu, India
Accepted Date: December 30 2012
Abstract
The discovery of biotinidase (BTD) deficiency, an inherited biotin-responsive disorder has increased the interest in biotinidase. Impaired serum BTD activity has been reported in patients suffering from chronic liver diseases, and in rats with experimentally induced acute or chronic liver injury. Molecular biology techniques such as tandem mass spectrometry have added a new dimension to our understanding of the role for biotinidase in cellular metabolism that go beyond the classical roles for biotinidase in the recycling of biotin. Recently, biotinidase has been implicated in the diagnosis of cancers. Decreased BTD is suggested as a potential serological biomarker for the detection of breast cancer .Recent reports suggests the potential applicability of biotinidase in the fine needle aspiration (FNA) diagnosis of aggressive papillary thyroid cancer. The exact role of biotinidase in cancer remains to be established. Histone biotinylation is considered to play an important role in signaling DNA damage. As biotinylation of histones depends on biotin supply it may be decreased in BTD deficient patients thereby affecting signaling of DNA damage. Elevated serum biotinidase activity has been reported in patients with glycogen storage diseases. The focus of this review is to update the metabolic importance of biotinidase and to present the applications of BTD in the diagnosis of liver diseases and recently, certain cancers.
Keywords
Biotinidase; breast cancer; cervical cancer; liver disease; thyroid cancer
Introduction
Biotinidase, the biotin recycling enzyme is ubiquitously distributed and occurs at high levels in the liver, serum, and kidney. It is is synthesized by the liver and secreted into the blood. The primary function of this enzyme is to cleave biotin from biocytin, thereby conserving biotin for use as a cofactor for four biotin-dependent carboxylases (propionyl CoA carboxylase (PCC), β-methylcrotonyl CoA carboxylase (MCC), pyruvate carboxylase (PC), and acetyl CoA carboxylase (ACC)) [1].
The carboxylases serve important roles in intermediary metabolism and their impairment can cause abnormalities in fatty acid synthesis, amino acid catabolism, and gluconeogenesis, resulting in the accumulation of abnormal organic acids. The absence or deficiency of biotinidase impairs the recycling of free biotin, thereby slowing the functioning of the biotin-dependent carboxylases.
Biotinidase, the biotin recycling enzyme belongs to the nitrilase superfamily of enzymes that consists of amidases, N-acyltransferases and nitrilases [2]. Some members of the nitrilase superfamily (vanins-1, -2 and -3) share significant sequence similarities with BTD [3]. The gene that encodes biotinidase is localized at 3p254 [4].
The discovery of biotinidase deficiency, an inherited biotin- responsive disorder that can be treated with biotin supplementation has increased the interest in biotinidase. Multiple carboxylase deficiency (MCD) responsive to biotin administration was first described in [5,6]. The infantile form of multiple carboxylase deficiency was characterised as biotinidase deficiency in the 1980s by Wolf et al. [7,8] which manifested as neurological and cutaneous lesions.
Biotinidase plays a fundamental role in biotin homeostasis
Biotin deficiency in humans is rare because biotin is continuously being recycled from the biotin dependent holocarboxylases by the action of biotinidase. Under normal conditions these enzymes undergo proteolytic degradation to biocytin or biotinyl peptides. Cleavage of these breakdown products by biotinidase recaptures and recycles the free biotin for continued cofactor functioning.
Biotinidase is also important for making biotin bioavailable from bound dietary sources. Biotin in the diet is mainly present in a protein-conjugated form. BTD is secreted in pancreatic fluids and plays a critical role in releasing free biotin from biocytin and biotinylated peptides prior to absorption. Intestinal BTD may also be derived from the intestinal flora, intestinal secretions and brush border membranes [9,10].
BTD participates in the transport of biotin to peripheral tissues [9].Thus, biotinidase plays a crucial role in maintaining the free biotin pool in the body and deficient biotinidase activity has been shown to result in late-onset MCD in children.
Biotinase may modify histones posttranslationally
In addition to hydrolytic activity, biotinidase has biotinyltransferase activity resulting in the transfer of biotin from biocytin to nucleophilic acceptors [11]. Biotinyl transferase activity occurs at physiological pH and at physiological concentrations of biocytin and, therefore, may be a major function of the enzyme in serum and other tissues. Recent studies have shown that biotinidase post translationally modifies histones by biotinylating them in the presence of biocytin and removing the biotinyl moiety from histones Pindolia et al [12].
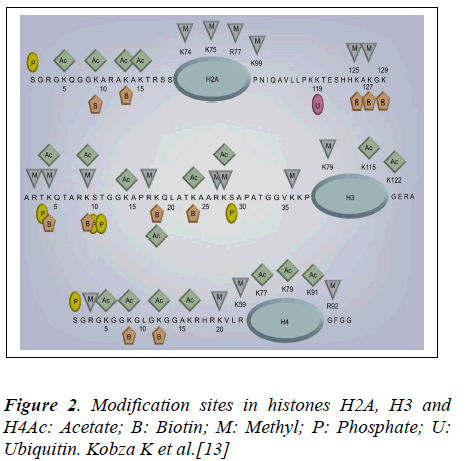
Eleven distinct biotinylation sites in histones H2A, H3and H4 (Figure 2) have been identified [13]. Although the specific role of histone biotinylation is not known, biotinylation of histones appears to participate in the biological processes such as gene expression, and DNA repair. Firstly, biotinylation of histones has been shown to increase (more than 4 fold) early in the cell cycle (G1 phase) [14] and remain increased during later phases (S, G2 and M phase) compared with quiescent controls. Fibroblasts from patients with holocarboxylase synthetase deficiency have severe deficiency in histone biotinylation [15] .It remains to be determined whether this is associated with decreased proliferation rates. Recently, it has been shown that biotinylation of distinct lysine residues in histone H4 changes at specific phases of the cell cycle [16].Secondly, studies in chicken erythrocytes have provided evidence that biotinylated histones are enriched in transcriptionally silent chromatin [17].
Figure 1: The biotin cycle and its roles. Free, unbound, biotin is the coenzyme for four carboxylases in humans and mice: propionyl-CoA carboxylase (PC), β- methylcrotonyl-CoA carboxylase (MCC), pyruvate carboxylase (PC), and acetyl-CoA carboxylase (ACC). Holocarboxylase synthetase (HCS) catalyzes the covalent attachment of free biotin to apocarboxylases forming holocarboxylases. The holocarboxylases participate in amino acid catabolism, fatty acid synthesis, and gluconeogenesis. Biotinidase releases biotin from biocytin and/or biotinylated peptides generated from the proteolytic turnover of holocarboxylases, thereby recycling biotin and resupplying the free biotin pool. Biotinidase also mediates the release of biotin from biocytin and/or biotinylated peptides generated by the proteolysis of dietary proteins. Biotinidase may posttranslationally modify histones by biotinylating them in the presence of biocytin and removing the biotinyl moiety from histones. HCS can also biotinylate histones and produces biotinyl-AMP, which can then participate in the cGMP signaling pathway regulating gene expression. Free biotin is absorbed in intestine, recaptured in the kidney and transported in the cytoplasm by transporter proteins, sodium-dependent multivitamin transporter (SMVT) and monocarboxylate transporter 1 (MCT1) Pindolia, et al [12].
Thirdly, it is proposed that biotinylation of histones might play a role in the cellular response to DNA damage Biotinylation of K12 in histone H4 is suggested to play roles in gene repression, DNA repair, heterochromatin structures and repression of transposons to mediate genomic stability and minimize cancer risk in human cells and Drosophila melanogaster [17,18]. Formation of thymine dimers caused by exposure of lymphoid cells to UV light, resulted in the increased biotinylation of Histones [17]. If double-stranded DNA breaks are caused by exposure of lymphoid and choriocarcinoma cells to etoposide, biotinylation of K12 in histone H4 shows a rapid and transient decrease [18] .This suggests a role for histone biotinylation in signaling DNA damage.
As biotinylation of histones depends on biotin supply [19] it may be decreased in BTD deficient patients where biotin recycling is impaired [20].
Tissue distribution of BTD
The liver is thought to be the source of serum biotinidase. Activities of this enzyme are high in the serum, liver, kidney and adrenal gland [21].
Cellular distribution of BTD
BTD activity is known to exist in microsomes and mitochondria [21,22], while the presence of BTD in nuclei is controversial. Pispa proposed that 26% of the cellular BTD activity is located in the nuclear fraction [21], which was confirmed by subsequent immunocytochemistry studies [23]. However, no nuclear BTD was detected by Stanley et al [24].
Metabolic consequences of biotinidase deficiency
A defect of biotinidase activity blocks biotin release from food or food recycling after carboxylase proteolysis. This result in a secondary biotin deficiency, disrupting the activities of all four biotin-dependent carboxylases (propionyl CoA carboxylase (PCC), β-methylcrotonyl CoA carboxylase (MCC), pyruvate carboxylase (PC), and acetyl CoA carboxylase (ACC)) [25]. Subsequently, gluconeogenesis, amino acid catabolism, and fatty acid synthesis are affected.
1. Pyruvate carboxylase, the enzyme that is involved in the catabolism of glucose, catalyzes the conversion of pyruvate to oxaloacetate within the citric acid cycle. Decreased activity of this enzyme results in the accumulation of lactic acid and alanine.
2. Propionyl-CoA carboxylase, the enzyme that is involved in the catabolism of specific branched-chain amino acids and odd-chain fatty acids, catalyzes the conversion of propionyl-CoA to methylmalonyl-CoA. Decreased activity of propionyl-CoA carboxylase results in the accumulation of propionate, methyl citric acid (MCA), and 3-hydroxypropionic acid (HPA).
3. The enzyme, 3-Methylcrotonyl-CoA carboxylase, involved in the catabolism of leucine, catalyzes the conversion of 3-methylcrotonyl-CoA to 3-methylglutaconyl- CoA. Deficient activity of 3-methylcrotonyl- CoA carboxylase (MCC) shunts the 3- methylcrotonyl-CoA to alternate metabolic pathways resulting in the accumulation of 3-hydroxyisoleric acid (3-HIVA), 3-methylcrotonylglycine (3-MCG), and isovalerylglycine (IVG). In humans, urinary excretion of 3-HIVA is considered the earliest, most sensitive indicator of biotin deficiency [26,27], whereas 3-HPA and 2- MCA are not [28].
4. The fourth carboxylase, acetyl-CoA carboxylase, located mainly in the cytosol and to a lesser extent in the mitochondrion, is involved in the regulatory step of fatty acid synthesis and catalyzes the conversion of acetyl-CoA to malonyl-CoA
Assay of Biotinidase enzyme activity
Biotinidase activity in serum is most commonly determined colorimetrically by measuring the release of paminobenzoate from N-biotinyl-p-aminobenzoate, a biocytin analog [29]. Biotinidase activity is also determined fluorimetrically by measuring the release of aminoquinoline from biotinyl-6-aminoquinoline[30]. Mean biotinidase activity in serum is 5.43 +/- 1.06 nmol/min/ml in normal adults [21].
In clinically symptomatic cases, urine analysis by gas chromatograph/mass spectrometry will identify elevation of ß-hydroxyisovalerate, lactate, ß-methylcrotonylglycine, ß-hydroxypropionate and methyl citrate, which build up due to the inactivation of the biotin requiring enzymes.
Inherited Biotinidase Deficiency
Biotinidase deficiency is a biotin-responsive, autosomal recessively inherited metabolic disorder resulting from mutations in the biotinidase gene [31]. Wolf has reported 150 mutations thus far [32] and depending upon the nature of the mutation(s), the enzyme deficiency maybe either complete or partial. Biotinidase deficiency is classified as either profound or partial based on the serum biotinyl-hydrolase enzyme activity (0–10% and 10–30%, respectively [33]. Despite its rarity, early recognition is crucial because expeditious treatment may reverse all of its manifestations [34].
The clinical symptoms of biotin deficiency can appear within a few weeks after birth or several years later. A wide spectrum of clinical manifestations, including abnormalities of the neurological, [35] dermatological, immunological, and ophthalmological systems has been reported. Symptoms include ataxia, hypotonia, developmental delay, conjunctivitis, skin rash and alopecia, seizures, hearing loss, breathing problems and optic atrophy. The expression of these symptoms is variable and may be related to dietary biotin intake and the degree of residual Biotinidase enzyme activity. If untreated, patients will develop metabolic ketoacidosis and organic aciduria. Most patients with Partial Biotinidase deficiency exhibit chiefly the cutaneous symptoms.
Biotinidase deficiency meets the major criteria for inclusion in newborn screening programs [33]. Essentially all newborn screening programs in the United States and many in other countries test for the disorder [36]. This allows neonates with biotinidase deficiency to be identified soon after birth and immediately treated with biotin, thereby preventing the development of symptoms [37]. Biotinidase deficiency can be detected in almost 100% of affected infants who participate in the newborn screening programme. The symptoms improve or can be prevented if affected children are treated with pharmacological doses of biotin .Failure to diagnose and treat BTD deficiency at an early stage may cause irreversible neurological damage, leading to developmental delay and autistic behavior [38,39].
BTD-deficient patients are treated with lifelong daily oral doses of biotin 5–20 mg, independent of their age to compensate for its decreased bioavailability from food sources and increased urinary losses [37].
Biotinidase activity in various diseases
Liver diseases
As biotinidase is synthesized in the liver [21] certain liver diseases can cause decreased synthesis of liver proteins or enzymes, including biotinidase, thereby reducing biotinidase activity in serum [40-44].
Nagamine et al [40] first reported that in various chronic liver diseases, biotinidase activities are significantly reduced as compared with healthy controls. These authors have also found a correlation with serum albumin, prothrombin time, and pseudocholinesterase respectively, suggesting that biotinidase activities may reflect the degree of liver damage. Low biotinidase activities have been reported in the sera of patients with impaired liver function [41].
In another study, Nagamine et al [42] have shown that serum biotinidase activity in decompensated liver cirrhosis (LC) and hepatoma is significantly lowered than in acute viral hepatitis (AVH), chronic viral hepatitis (CVH), and compensated LC. Pabuçcuoğlu et al [43] report significantly lower serum biotinidase activity in patients with cirrhosis, particularly in the patients with decompensated cirrhosis and fulminant hepatitis. The decreased serum biotinidase activity in chronic liver diseases is attributed to severe impairment of hepatocellular function. Report from our laboratory has shown that Biotinidase activity is significantly lowered in the serum of patients with acute and chronic liver disease [44]. Using receiver-operator characteristic curves, serum biotinidase was found to have high values of sensitivity and specificity when applied as a diagnostic test in both acute and chronic liver disease. These results suggest that serum biotinidase may be a sensitive and specific diagnostic marker of hepatic biosynthetic function in both acute and chronic liver disease. In a recent study Hayakawa and Nagamine have demonstrated altered biotinidase kinetics in human hepatocellular carcinoma [45].
Liver injury
It has been shown previously that epileptic patients receiving a high average dose of anticonvulsants (valproic acid) have lower biotin concentrations than those receiving a low dose [46]. Liver function data were found to be elevated in the patients receiving low dose and high dose of valproic acid. On the other hand, biotinidase activity was significantly statistically lowered as compared with controls. It is suggested that VPA impairs the liver mitochondrial function, resulting in a low biotinidase activity and biotin deficiency. In our laboratory, we have investigated the effect of hepatotoxins on the plasma biotinidase activity in rats. We observed a marked decrease in the activity of biotinidase in the plasma of rats after single dose of carbon tetrachloride [47]. Liver fibrosis induced in rats by chronic exposure to carbon tetrachloride resulted in a significant decrease in plasma biotinidase activity, while the plasma albumin levels were normal [48,49].
Acute acetaminophen toxicity in rats results in increased plasma biotinidase activity. In the plasma of ethanol/ phenobarbital-treated rats as well as the ethanoltreated rats, albumin and the activity of biotinidase were decreased significantly as compared with the controls [50].
Glycogen storage diseases
An elevated serum biotinidase activity in patients with glycogen storage disease (GSD) type Ia has been reported previously. It has been suggested that GSD type Ia should be considered in children with markedly elevated serum biotinidase activity [51,52].
Paesold-Burda et al [53] reported increased biotinidase activity in patients with various glycogen storage disorders- GSD Ia, GSD I non-a, GSD III, GSD VI and GSD IX. The sensitivity of this test was 100% for patients with GSD Ia, GSD I non-a and GSD VI, 62% for GSD III, and 77% for GSD IX respectively. These authors propose serum biotinidase as a diagnostic biomarker for hepatic glycogen storage disorders. The reason why plasma BTD activity increases in GSDs patients or the clinical importance of the increment is not known.
Breast cancer
Recently BTD has been suggested as a potential serological biomarker for the detection of breast cancer. Kang et al, [54] using a blind set of plasmas obtained from 21 breast cancer patients and 21 normal healthy controls, have confirmed that BTD is significantly down-regulated in breast cancer plasma. BTD levels were lowered in all cancer grades (I-IV). These authors have suggested that BTD may be a potential serological biomarker for the detection of breast cancer.
Thyroid tumors
In an attempt to identify thyroid cancer biomarkers, Kashat et al [55] analysed proteins secreted by thyroid cancer cell lines, papillary-derived TPC-1 and anaplasticderived CAL62, by liquid chromatography-tandem mass spectrometry. Biotinidase was one among those proteins detected in thyroid cancer patients' sera, suggesting its potential as blood-based thyroid cancer marker. Subsequently, Anthony et al [56] analysed the expression of biotinidase in thyroid cancer tissues and fine needle aspiration (FNA) samples to evaluate its diagnostic and prognostic potential in thyroid cancer.
Immunohistochemical analysis of biotinidase expression showed an overall decrease in papillary thyroid carcinoma (PTC) compared to benign tissue. In addition, aggressive PTC showed a decrease in overall biotinidase expression when compared with non-aggressive PTC. Loss of overall biotinidase expression was associated with poor disease free survival. These authors examined the effect of subcellular distribution of biotinidase on patient survival. Decreased nuclear expression of biotinidase was observed in PTC as compared to benign tissue. These authors have suggested that loss of biotinidase expression may have applicability as a potential diagnostic marker for FNA samples with inconclusive diagnosis and that it can potentially reduce unnecessary thyroid resections. The role biotinidase plays in cancer aggressiveness remains to be established. One probable hypothesis is the role of biotinidase in the recycling of biotin which acts as a co-factor for enzymes responsible for chromatin structure and stability. Biotinidase cleaves biocytin thereby making free biotin readily available. It could be speculated that biotin deficiency might lead to critical epigenetic alterations in cancer attributing an aggressive phenotype to it in the process. Whether the loss of biotinidase plays a functional role or is associated with cancer aggressiveness remains to be addressed
Cervical carcinoma
Huang et al [57] identified a gene expression profile consisting of 11 genes that could predict pelvic lymph node metastasis in cervical carcinoma using oligonucleotide microarray. Surprisingly, one of the genes in their panel was biotinidase, down regulated in pelvic lymph node metastasis, akin to our findings in thyroid cancer.
Possible role of altered Biotinidase expression in various cancers
Evidence suggests that biotin is also important in cell signaling, gene expression, and chromatin structure. It is suggested that biotin-dependent gene products play important role in signal transduction, gene expression, and cell proliferation and differentiation .More than 2000 human genes depend on biotin for expression. One such process is regulation of transcription factor NF-α -B, which is important in prevention of all death [58]. Evidence has been provided that expression of N-myc, cmyb, N-ras and raf oncogenes depends on biotin in small cell lung cancer cells [59].
Loss of biotinidase would subsequently cause a biotin deficient state which would in turn affect histone biotinylation in chromatin remodeling. Eleven distinct biotinylation sites in histones H2A, H3 and H4 have been identified (Figure 2). It is known that biotinylation of K12 in histone H4 is important for repair of DNA and heterochromatin structures as well as repression of genes and transposons to maintain genomic stability and reduce cancer risk in human cells and Drosophila melanogaster [18].
Conclusion
In the present review, the importance of biotinidase, consequences of its deficiency, and its implications in the diagnosis of cancer is reviewed. Serum biotinidase activity has been used as a marker of hepatic synthetic ability. Its activity is reported to be decreased in patients with acute or chronic liver damage as well as in rats with liver injury. Recent studies have suggested the usefulness of biotinidase in the diagnosis of cancers including breast cancer, papillary thyroid carcinoma and cervical cancer. Whether the loss of biotinidase plays a functional role or is associated with cancer remains to be investigated. Additional work in this area could shed crucial light on the role of biotinidase in cancers.
References
- Camporeale, G.; Zempleni, J. Biotin. In: Bowman, BA; Russell, RM., editors. Present Knowledge in Nutrition. International Life Sciences Institute; Washington, DC, USA: 2006. p. 314-326.
- Brenner C. Catalysis in the nitrilase superfamily. Curr Opin Struct Biol 2002; 12:775-782.
- Maras B, Barra D, Dupre S, Pitari G. Is pantetheinase the actual identity of mouse and human vanin-1 proteins? FEBS Lett 1999; 461:149-152.
- Cole H, Reynolds TR, Lockyer JM, et al. Human serum biotinidase cDNA cloning, sequence, and characterization. J Biol Chem 1994; 269:6566-6570.
- Gompertz D, Draffan GH, Watts JL, Hull D. Biotinresponsive beta-methylcrotonyl glycinuria. Lancet 1971; 2: 22-24.
- Wolf B, Hsia YE, Sweetman L, et al. Multiple carboxylase deficiency: clinical and biochemical improvement following neonatal biotin treatment. Pediatrics 1981; 68: 113-8.
- Wolf B, Heard GS, Weissbecker KA, et al. Biotinidase deficiency: initial clinical features and rapid diagnosis. Ann Neurol. 1985; 18: 614-617.
- Tsao CY, Kien CL. Complete biotinidase deficiency presenting as reversible progressive ataxia and sensorineural deafness. J Child Neurol. 2002; 17:146.
- Wolf B, Grier RE, McVoy JRS, Heard GS. Biotinidase deficiency: a novel vitamin recycling defect. J Inherit Metab Dis 1985; 8: 53-58.
- Bowman BB, Rosenberg I. Biotin absorption by distal rat intestine. J Nutr 1987; 117:2121–2126.
- Ballard TD, Wolff J, Griffin JB, et al. Biotinidase catalyzes debiotinylation of histones. Eur J Nutr 2002; 41:78-84.
- Pindolia, K., Chen J, Cardwell C, Cui X, Chopp M, Wolf B. Neurological deficits in mice with profound biotinidase deficiency is associated with demyelination and axonal degeneration. Neurobiol Dis 2012; 47: 428-35.
- Kobza K, Sarath G, Zempleni J. Prokaryotic BirA ligase biotinylates K4, K9, K18 and K23 in histone H3. BMB Reports 2008; 41: 310-315.
- Stanley JS, Griffin JB, Zempleni J. Biotinylation of histones in human cells: effects of cell proliferation. Eur J Biochem 2001; 268: 5424-5129.
- Narang MA, Dumas R, Ayer LM, Gravel RA. Reduced histone biotinylation in multiple carboxylase deficiency patients: a nuclear role for holocarboxylase synthetase. Hum Mol Genet 2004; 13: 15-23.
- Kothapalli N, Zempleni J. Biotinylation of histones depends on the cell cycle in NCI-H69 small cell lung cancer cells. FASEB J 2005; 19:A55.
- Peters DM, Griffin JB, Stanley JS, Beck MM, Zempleni J. Exposure to UV light causes increased biotinylation of histones in Jurkat cells. Am J Physiol Cell Physiol 2002; 283: C878-884.
- Kothapalli N, Sarath G, Zempleni J. Kothapalli N, and Zempleni J. Biotinylation of K12 in histone H4 decreases in response to DNA double-strand breaks in human JAr choriocarcinoma cells. J Nutr. 2005; 135: 2337-242.
- Smith EM, Hoi JT, Eissenberg JC, et al. Feeding Drosophila a biotin-deficient diet for multiple generations an increase stress resistance and lifespan and alters gene expression and histone biotinylation patterns. J Nutr 2007; 137: 2006-2012
- Camporeale G, Zempleni J, Eissenberg JC. Susceptibility to heat stress and aberrant gene expression patterns in holocarboxylase synthetase-deficient Drosophila melanogaster are caused by decreased biotinylation of histones, not of carboxylases. J Nutr 2007; 137: 885–889.
- Pispa J. Animal biotinidase. Ann Med Exp Biol Fenniae 1965; 43: 4-39.
- Garganta CL, Wolf B. Lipoamidase activity in human serum is due to biotinidase. Clin Chim Acta 1990; 189: 313-325.
- Chew YC, Sarath G, Zempleni J. An avidin-based assay for quantification of histone debiotinylase activity in nuclear extracts from eukaryotic cells. J Nutr Biochem 2007; 18: 475-481.
- Stanley CM, Hymes J, Wolf B. Identification of alternatively spliced human biotinidase mRNAs and putative localization of endogenous biotinidase. Mol Genet Metab 2004; 81: 300-312.
- Wolf, B., Feldman, G.L., 1982. The biotin-dependent carboxylase deficiencies. Am. J. Hum. Genet. 34, 699- 716.
- Stevenson DE, Feng R, Dumas F, Groleau D, Mihoc A, Storer AC. Mechanistic and structural studies on Rhodococcus ATCC 39484 nitrilase. Biotechnol Appl Biochem 1992; 15: 283-302.
- Mock DM, Heinrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validation of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am.J.Clin.Nutr 2002; 76: 1061-1068.
- Mock DM, Henrich-Shell CL, Carnell N, Stumbo P, Mock NI. 3-Hydroxypropionic acid and methyl citric acid is not reliable indicators of marginal biotin deficiency in humans. J.Nutr 2004; 134: 317-320.
- Wolf B, Grier RE, Allen RJ, and Goodman SI, Kien CL. Biotinidase deficiency: the enzymatic defect in late onset multiple carboxylase deficiency. Clin Chim Acta. 1983a; 131: 273-281
- Wastell H, Dale G, Bartlett K. A sensitive fluorimetric rate for biotinidase using a new derivative of biotin, biotinyl-6-aminoquinoline. Anal Biochem. 1984; 140: 69-73
- Pomponio RJ, Narasimhan V, Reynolds TR, et al. Deletion/ insertion mutation that causes biotinidase deficiency may result from the formation of a quasipalindromic structure. Hum Mol Genet 1996; 5: 1657-1661
- Wolf B, Jensen K, Huner G, et al. Seventeen novel mutations that cause profound biotinidase deficiency. Mol Genet Metab 2002; 77: 108-111
- Wolf B. Worldwide survey of neonatal screening for biotinidase deficiency. J Inher Metab Dis 1991; 14: 923-927
- Dahiphale R, Jain S, Agrawal M. Biotinidase deficiency. Indian Pediatr. 2008; 45: 777-779
- Wolf B. The neurology of biotinidase deficiency Minireview .Molecular Genetics and Metabolism 2011; 104: 27-34
- Wolf B, Heard GS. Screening for biotinidase deficiency in newborns: worldwide experience, Pediatrician 1990; 85: 512-517.
- Baumgartner ER, Suormala T. Multiple carboxylase deficiency: inherited and acquired disorders of biotin metabolism. Int J Vitam Nutr Res 1997; 67: 377-384.
- Tsao CY, Kien CL. Complete biotinidase deficiency presenting as reversible progressive ataxia and sensorineural deafness. J Child Neurol. 2002; 17: 146.
- Zaffanello M, Zamboni G, Fontana E, Zoccante L, Tato L. A case of partial biotinidase deficiency associated with autism. Child Neuropsychol 2003; 9: 184-188.
- Nagamine T, Saito S, Yamada S, Kaneko M, Uehara M, Takezawa J, Kobayashi S, Oizumi J, Iinuma K. Clinical evaluation of serum biotin levels and biotinidase activities in patients with various liver diseases.[ Article in Japanese] Nihon Shokakibyo Gakkai Zasshi. 1990; 87: 1168-1174.
- Grier RE Heard GS, Watkins P, Wolf B. Clin Chim Acta. 1990; 186: 397-400.
- Nagamine T, Saito S, Yamada S, Arai T, Takehara K, Fukui T. Biotinidase activity in patients with liver disease. Scand J Gastroenterol. 1993; 28: 899-906
- Pabuçcuoğlu A, Aydoğdu S, Bas M. Serum biotinidase activity in children with chronic liver disease and its clinical significance. J Pediatr Gastroenterol Nutr. 2002; 34: 59-62.
- Faith M, Eapen CE, Wilfred G, Ramachandran J, Jacob M. Serum biotinidase is a sensitive and specific biochemical marker of hepatic dysfunction: A preliminary report. Hepatol Res. 2007; 37: 13-17
- Hayakawa K and Nagamine T. Effect of Fucoidan on the Biotinidase Kinetics in Human Hepatocellular Carcinoma. Anticancer Research 2009; 29: 1211-1218.
- Schulpis KH, Karikas GA, Tjamouranis J, Regoutas S, Tsakiris S. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001; 42: 1359-1362.
- Abraham P, Wilfred G. A marked decrease in the activity of biotinidase in the plasma of rats after single dose of carbon tetrachloride. Clinica Chimica Acta 2003; 328: 195-197.
- Abraham P, Wilfred G, Ramakrishna B. Decrease in plasma biotinidase activity with normal albumin concentrations in experimental liver fibrosis. Clinica Chimica Acta 2003; 334: 245-247.
- Abraham P. Increased plasma biotinidase activity in rats with paracetamol induced acute liver injury. Clinica Chimica Acta 2004; 349: 61-65
- Abraham P, Wilfred G, Ramakrishna B. Oxidative damage to the hepatocellular proteins after chronic ethanol intake in the rat. Clin Chim Acta. 2002; 325:117-125.
- Wolf B, Freehauf CL, Thomas JA, Gordon PL, Greene CL, Ward JC. Markedly elevated serum biotinidase activity may indicate glycogen storage disease type Ia. J Inherit Metab Dis. 2003; 26: 805-9.
- Angaroni CJ, Giner-Ayala AN, Hill LP, Guelbert NB, Paschini-Capra AE, Dodelson de Kremer R. Evaluation of the biotinidase activity in hepatic glycogen storage disease patients. Undescribed genetic finding associated with atypical enzymatic behavior: an outlook. J Inherit Metab Dis. 2010; 33: S289-294.
- Paesold-Burda P, Baumgartner MR, Santer R, Bosshard NU, Steinmann B. Elevated serum biotinidase activity in hepatic glycogen storage disorders—a convenient biomarker. J Inherit Metab Dis. 2007; 30: 896-902.
- Kang UB, Ahn Y, Lee JW, Kim YH, Kim J, Yu MH, Noh DY, Lee C. Differential profiling of breast cancer plasma proteome by isotope-coded affinity tagging method reveals biotinidase as a breast cancer biomarker. BMC Cancer. 2010; 10: 114
- Kashat L, So AK, Masui O, Wang XS, Cao J, et al. Secretome-based identification and characterization of potential biomarkers in thyroid cancer. J Proteome Res 2010; 9: 5757-5769.
- Anthony KC. Jatinder K. Ipshita K. Jasmeet. A et al. Biotinidase is a Novel Marker for Papillary Thyroid Cancer Aggressiveness. PLoS One. 2012; 7: e40956.
- Huang L, Zheng M, Zhou QM, Zhang MY, Jia WH, Yun JP, Wang HY. Identification of a gene-expression signature for predicting lymph node metastasis in patients with early stage cervical carcinoma. Cancer. 2011; 117: 3363-3373
- Li X, Stark GR. NF B-dependent signaling pathways. Exp Hematol 2002; 30: 285-296.
- Scheerger SB, Zempleni J. Expression of oncogenes depends on biotin in human small cell lung cancer cells NCI-H69. Int J Vitam Nutr Res 2003; 73: 461-467.