Research Article - Journal of Dermatology Research and Skin Care (2024) Volume 8, Issue 1
An Open-Label, Randomized Clinical trial of Psorolin B ointment in the treatment of plaque psoriasis
Amruthavalli GV*, Aruna V, Gayathri Rajagopal
Dr JRK’s Research and Pharmaceuticals Pvt., Ltd, Chennai, India
- *Corresponding Author:
- Amruthavalli GV
Dr JRK’s Research and Pharmaceuticals Pvt.
Ltd, Chennai, India
E-mail: amruthavalli_gv@jrkresearch.com
Received: 10-Jan-2024, Manuscript No. AADRSC-24-124888; Editor assigned: 12-Jan-2024, PreQC No. AADRSC-24-124888 (PQ); Reviewed: 29-Jan-2024, QC No AADRSC-24-124888; Revised: 02-Feb-2024, Manuscript No. AADRSC-24-124888 (R); Published: 10 -Feb-2024, DOI:10.35841/aadrsc-8.1.186
Citation: Amruthavalli GV. An Open-Label, Randomized Clinical trial of Psorolin B ointment in the treatment of plaque psoriasis. Dermatol Res Skin Care. 2024; 8(1):186
Keywords
Psorolin B ointment, ointment for psoriasis, Clinical trial, Psoriasis ointment, Anti-inflammatory and anti-scaling.
Introduction
Psoriasis is an incurable auto-immune disorder of the skin with intermittent inflammatory manifestation resulting in causing acute suffering to the patient [1]. Several drugs are although available for the treatment of psoriasis but still a treatment that would offer great solace to the patient is far from near. Steroidal preparations, keratinolytic agents, vitamin D analogues and high end mutagenic agents etc., are used in the treatment of psoriasis with limited treatment benefit but often comes with severe side effects [2].
In the last three decades, at least in India, several herbal preparations have occupied an exalted position in the treatment of psoriasis [3] but mostly the treatment is limited to maintain the remission phase than for managing the inflammatory response of the disease.
Among various herbal preparations, the role of Wrightia tinctoria leaf oil has shown remarkable therapeutic value in the treatment of psoriasis [4]. Subsequently the inclusion of many other herbs in the treatment armamentarium of psoriasis have arrived and following are a few plants to name such as Boswellia serrata, Indigofera tinctoria, Hydnocarpus laurifolia, Aloe vera etc.
Psorolin B is an advanced psoriasis specialist ointment, the proprietary Siddha medicine of Dr.JRK’s research and Pharmaceuticals Pvt., Ltd., Chennai. The formulation has been studied in detail for its pharmacological effect with reference to histidine decarboxylase inhibition, keratinocyte suppression, anti-oxidant effect, collagen and elastin protection effect through suppressing collagenase and elastase enzymes [5-9].
The present study describes the findings of the clinical trial of Psorolin B ointment in the treatment of plaque psoriasis. The clinical trial was done as per Helsinki declaration 1979. Findings are presented in the article.
Materials and Methods
Description of the investigational drug
Psorolin B ointment is composed of : 33.3%
Oil extract of Wrightia tinctoria :3.33%
Oil extract of Cynodon dactylon: 3.33%
Boswellia serrata: 1%
Hydnocarpus laurifolia: 0.1%
Red ochre :0.2% and the ointment base contains wheat germ oil, Vitamin E, Natural source of Vitamin D and Salicylic acid besides the ingredients used to thicken the base.
Patient details
Patients with plaque psoriasis, willing to take part and who met the eligibility criteria were enrolled in the study. Prior to enrolment, screening was done based on strict inclusion and exclusion criteria.
A total of 20 patients were included in the study.
Post recruitment, informed consent was obtained from each patient and then the patients were reviewed on the following days
Visit 1 Day 0, Visit 2 – Day 28 ± 2, Visit 3 – Day 56 ± 3, Visit – Visit 4 – Day 84 ± 3
Inclusion criteria
• Both gender, between 18-60 years of age
• Patients with stable plaque psoriasis
• Patients with psoriasis involvement less than 10% of total body surface.
(The percentage of psoriasis involvement was calculated in terms of hand units. One hand unit is approximately equivalent to 1% of the total body surface area).
• Patients with the category of disease under “mild or moderate” on Investigator’s Global Assessment (IGA) Scale.
• Patients with treatment area amenable to topical treatment and easy for clinical assessment.
• Patients with lesions at bilateral anatomical sites and the lesion size of 2.5 x 2.5 cm (~6 cm2) either on the trunk, arms or legs with a Target Plaque Severity Score (TPSS) ≥ 4 to ≤ 6 and induration sub score = 2.
• Female patients of childbearing potential
• Patients whose screening laboratory values are within normal limits or considered by the physician or Principal/ Clinical Investigator to be of no clinical significance.
• Patients who have no clinically significant abnormalities on the skin during physical examination.
• Patients who have no clinically significant abnormalities during physical examination (including but may not be limited to an evaluation of the cardiovascular, gastrointestinal, respiratory, musculoskeletal and central nervous system) and vital sign assessments.
• Patients willing to give written informed consent and adhere to all requirements of the protocol.
Exclusion Criteria
• Patients with known hypersensitivity or allergic skin.
• Pregnant or lactating females.
• Patients currently having non-plaque forms of psoriasis eg: erythrodermic, guttate, or pustular psoriasis or a drug induced form of psoriasis.
• Patients with systemic treatment with immunosuppressive drugs or corticosteroids within 4 weeks prior to enrolment.
• Patients with systemic treatment with retinoids within 8 weeks prior to enrolment.
• Patients with topical treatment with immune-modulators or corticosteroids within 4 weeks prior to enrolment.
• Patients with phototherapy treatment within 4 weeks prior to enrolment.
• Patients with other topical therapy on the treatment area within 1 week prior to enrolment.
• Patients requiring use of anti-histamine treatment during the study.
• Patients with clinical infection on the treatment area.
• Patients with presence or history of cancer including skin cancer.
• Patients with evidence of skin conditions other than psoriasis that would interfere with evaluations of the effect of study medication on psoriasis.
• Patients with presence or history of an immunocompromised disease.
• Patients with intense sun exposure during the study.
• Patients known or suspected of not being able to comply with the trial protocol (e.g. alcoholism, drug dependency, or psychotic state).
• Patients with history of severe cardiac, pulmonary, hepatic, renal, neurologic disease or mental illness.
• Patients with any medical condition or use of any medications that, in the opinion of the Principal Investigator, should preclude participation.
• Patients who have participated in a clinical study in the past 30 days or current participation in another clinical study.
• Patients with history of severe cardiac, pulmonary, hepatic, renal, neurologic disease or mental illness.
• Patients with any medical condition or use of any medications that, in the opinion of the Principal Investigator, should preclude participation.
• Patients who have participated in a clinical study in the past 30 days or current participation in another clinical study.
Usage instruction of Psorolin B ointment
Patients were instructed to apply the ointment as a thin layer over the psoriatic lesion and massage gently until the ointment is completely absorbed.
Efficacy evaluation
Response was assessed on 6-point scale, 5 = complete clearance (100% improvement); 4 = excellent (75-99% improvement); 3 = good (50-74% improvement); 2 = fair (25-49% improvement); 1 = poor (1-24% improvement); 0 = lesion remained unchanged or worsened.
Primary Outcome Measure
Patients were evaluated by global response to treatment for the target lesion based on TPSS Individual score of the following clinical measures such as erythema, induration and scaling was recorded during assessment.
Safety evaluation
Safety aspect was assessed based on tolerability, clinical and laboratory parameters, vital signs and physical examinations.
• Incidence of treatment emergent AEs [Time Frame: Baseline to Week 12]
• Patients rating of skin dryness on a 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) [Time Frame: Baseline to Week 4, 8 and 12].
• Patients rating of skin pruritus on a 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) [Time Frame: Baseline to Week 4, 8 and 12].
Patients rating of skin burning/stinging on a 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) [Time Frame: Baseline to Week 4, 8 and 12].
• Subject's Assessment of Tolerability of Treatment
Tolerability of treatment as assessed by subject will be evaluated on the Five point categorical:
Scale: - excellent, - very good, - good, - fair, - poor.
Results
Demographic and other baseline details
The present study was conducted in 20 patients with plaque psoriasis of which 9 were males and 11 were females. The mean age, weight, height and body mass index (BMI) of the study population was 44.1 years (range 23-59 years), 68.75 kg (range 58.0-81.0 kg), 168.75cm (160.0-176.0 cm) and 24.20 kg/ m2 (range 20.10-27.00 kg/m2), respectively. The demographic details of the patients are summarized in Table 1.
| Subject No. | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|
| S01 | 23 | 166 | 58 | 21 |
| S02 | 54 | 170 | 66 | 22.8 |
| S03 | 43 | 168 | 60 | 21.3 |
| S04 | 40 | 166 | 58 | 21 |
| S05 | 39 | 172 | 70 | 23.7 |
| S06 | 44 | 160 | 68 | 26.6 |
| S07 | 33 | 162 | 70 | 26.7 |
| S08 | 35 | 160 | 68 | 26.6 |
| S09 | 59 | 175 | 81 | 26.1 |
| S10 | 57 | 166 | 70 | 25.4 |
| S11 | 41 | 176 | 80 | 25.8 |
| S12 | 53 | 160 | 68 | 26.6 |
| S13 | 48 | 168 | 60 | 21.3 |
| S14 | 50 | 172 | 80 | 27 |
| S15 | 50 | 175 | 76 | 24.8 |
| S16 | 48 | 170 | 66 | 22.8 |
| S17 | 31 | 175 | 76 | 24.81 |
| S18 | 43 | 174 | 76 | 25.1 |
| S19 | 52 | 176 | 58 | 20.1 |
| S20 | 39 | 164 | 66 | 24.5 |
| Mean | 44.10 | 168.75 | 68.75 | 24.20 |
| SD | 9.27 | 5.60 | 7.57 | 2.28 |
| MIN | 23.00 | 160.00 | 58.00 | 20.10 |
| Max | 59.00 | 176.00 | 81.00 | 27.00 |
| %cv | 21.01 | 3.32 | 11.02 | 9.43 |
Table 1. Demographic and other baseline details
Target Plaque Severity Score
Mean TPSS (Target Plaque Severity Score) in Psorolin B Ointment group was 2.95 ± 0.22 and the median TPSS in Psorolin B Ointment was “3”
Approximately 95% of patients in Psorolin B ointment group reported good improvement (Score = 3) and no patient in the group reported poor improvement to Psorolin B treatment.
The treatment response - TPSS to Psorolin B treatment was statistically significant (*p = <.0001).
Mean TPSS (Mean ± SD) at visit 1 in Psorolin B ointment group was 5.60 ± 0.82
Statistically significant ($p = <.0001) improvement in TPSS was observed from baseline to visit 4 in Psorolin B ointment group
Mean TPSS (Mean ± SD) at visit 4 in Psorolin B ointment group was 1.25 ± 0.55
Statistically significant (*p = <.0001) improvement in TPSS was observed in Psorolin B ointment at visit 4 Table 2 Table 3.
| Global Response TPSS - Psorolin B Ointment | |
|---|---|
| N | 20 |
| Mean | 2.95 |
| SD | 0.22 |
| Min | 2 |
| Max | 3 |
| Median | 3 |
| CV | 7.58 |
Table 2. Global Response TPSS - Psorolin B Ointment
| TPSS | Day 0 (Visit 1) (Baseline) |
Assessment Visit 1 (Visit 2) |
Assessment Visit 2 (Visit 3) |
End of treatment visit (Visit 4) |
|---|---|---|---|---|
| Psorolin B Ointment | ||||
| N | 20 | 20 | 20 | 20 |
| Mean | 5.60 | 3.65 | 1.75 | 1.25 |
| SD | 0.82 | 1.18 | 0.72 | 0.55 |
| Min | 4 | 1 | 1 | 1 |
| Max | 8 | 5 | 3 | 3 |
| Median | 6 | 4 | 2 | 1 |
| CV | 14.66 | 32.39 | 40.93 | 44.01 |
| p Value (Paired T Test) Each visit compared with baseline | <.0001 | <.0001 | <.0001 | |
Table 3. Target Plaque Severity Score (TPSS) with Psorolin B Ointment
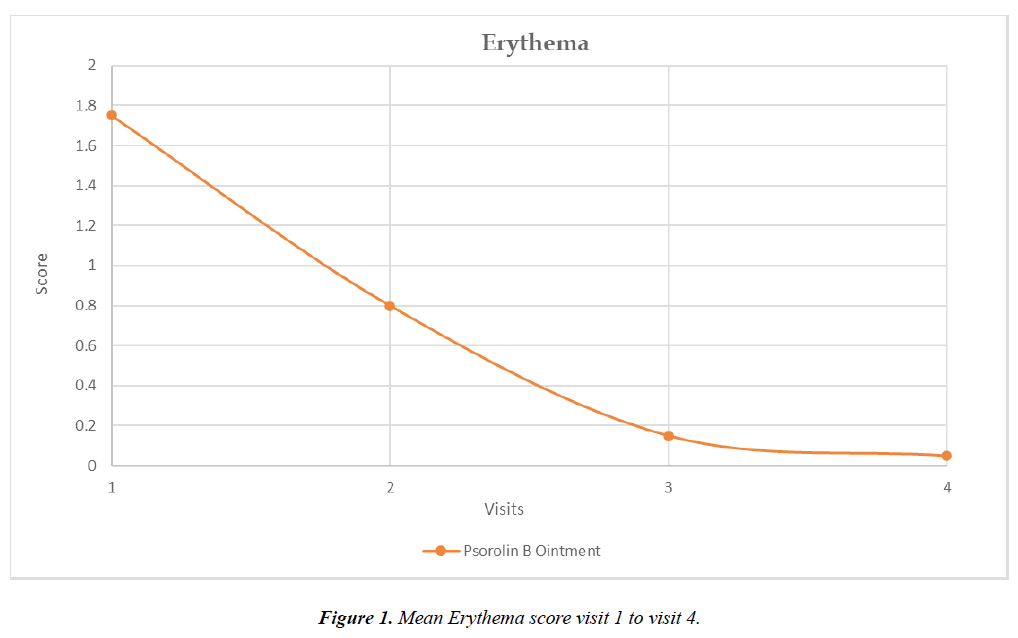
Erythema:
Mean Erythema score (Mean ± SD) at visit 1 in Psorolin B ointment group was 1.75 ± 0.55
Mean Erythema score (Mean ± SD) at visit 4 in Psorolin B ointment group was 0.05 ± 0.22
Percentage reduction of Erythema score from baseline to visit 4 in Psorolin B ointment group was 97.14%.
Statistically significant (*p = <.0001) improvement in erythema score was observed in Psorolin B ointment group at visit 4.
Graph 1: Mean Erythema score visit 1 to visit 4 Figure 1
Induration / Thickness:
Mean Induration / Thickness score (Mean ± SD) at visit 1 in Psorolin B ointment group was 2.10 ± 0.31
Mean Induration / Thickness score (Mean ± SD) at visit 4 in Psorolin B ointment group was 0.95 ± 0.22
Percentage reduction of Induration / Thickness score from baseline to visit 4 for Psorolin B ointment group was 54.76%
Statistically significant (*p = 0.0002) improvement in induration / thickness score was observed in Psorolin B ointment group at visit 4 Table 4.
| Induration / Thickness | Day 0 (Visit 1) (Baseline) |
Assessment Visit 1 (Visit 2) |
Assessment Visit 2 (Visit 3) |
End of treatment visit (Visit 4) |
|---|---|---|---|---|
| Psorolin B Ointment | ||||
| N | 20 | 20 | 20 | 20 |
| Mean | 2.10 | 1.60 | 1.00 | 0.95 |
| SD | 0.31 | 0.50 | 0.00 | 0.22 |
| Min | 2 | 1 | 1 | 0 |
| Max | 3 | 2 | 1 | 1 |
| Median | 2 | 2 | 1 | 1 |
| CV | 14.66 | 31.41 | 0.00 | 23.54 |
| p Value (Paired T Test) Each visit compared with baseline | <.0001 | <.0001 | <.0001 | |
| Percentage Change at Visit 4 from Baseline | - 54.76 % | |||
Table 4. Induration / Thickness - Psorolin B Ointment
Scaling:
Mean scaling score (Mean ± SD) at visit 1 in Psorolin B ointment group was 1.85 ± 0.59
Mean scaling score (Mean ± SD) at visit 4 in Psorolin B ointment group was 0.25 ± 0.44
Percentage reduction of scaling score from baseline to visit 4 for Psorolin B ointment group was 86.49%
Statistically significant (*p = 0.0002) improvement in scaling was observed in Psorolin B ointment group at visit 4 Table 5.
| Scaling | Day 0 (Visit 1) (Baseline) |
Assessment Visit 1 (Visit 2) |
Assessment Visit 2 (Visit 3) |
End of treatment visit (Visit 4) |
|---|---|---|---|---|
| Psorolin B Ointment | ||||
| ssN | 20 | 20 | 20 | 20 |
| Mean | 1.85 | 1.20 | 0.60 | 0.25 |
| SD | 0.59 | 0.52 | 0.50 | 0.44 |
| Min | 1 | 0 | 0 | 0 |
| Max | 3 | 2 | 1 | 1 |
| Median | 2 | 1 | 1 | 0 |
| CV | 31.74 | 43.60 | 83.77 | 177.70 |
| p Value (Paired T Test) Each visit compared with baseline | <.0001 | <.0001 | <.0001 | |
| Percentage Change at Visit 4 from Baseline | - 86.49 % | |||
Table 5. Scaling - Psorolin B Ointment
Safety Evaluation
Skin Dryness:
Mean skin dryness (Mean ± SD) at visit 1 in Psorolin B ointment group was 1.85 ± 0.49
Mean skin dryness (Mean ± SD) at visit 4 in Psorolin B ointment group was 0.11
Statistically significant (*p = <.0001) improvement in skin dryness was observed in Psorolin B ointment group at visit 4.
Skin Pruritus:
Mean Skin Pruritus (Mean ± SD) at visit 1 in Psorolin B ointment group was 1.85 ± 0.49
Mean Skin Pruritus (Mean ± SD) at visit 4 in Psorolin B ointment group was 0.84 ± 0.37
Statistically significant (*p = 0.0120) improvement in skin pruritus was observed in Psorolin B ointment group at visit 4 Table 6.
| Skin Pruritus | Day 0 (Visit 1) (Baseline) |
Assessment Visit 1 (Visit 2) |
Assessment Visit 2 (Visit 3) |
End of treatment visit (Visit 4) |
|---|---|---|---|---|
| Psorolin B Ointment | ||||
| N | 20 | 20 | 20 | 19 |
| Mean | 1.85 | 1.25 | 0.90 | 0.84 |
| SD | 0.49 | 0.44 | 0.45 | 0.37 |
| Min | 1 | 1 | 0 | 0 |
| Max | 3 | 2 | 2 | 1 |
| Median | 2 | 1 | 1 | 1 |
| CV | 26.45 | 35.54 | 49.69 | 44.49 |
| p Value (Paired T Test) Each visit compared with baseline | <.0001 | <.0001 | <.0001 | |
Table 6. Skin Pruritus - Psorolin B Ointment
Skin Burning / Stinging:
Mean Skin Burning / Stinging (Mean ± SD) at visit 1 in Psorolin B ointment group was 1.65 ± 0.49
Mean Skin Burning / Stinging (Mean ± SD) at visit 4 in Psorolin B ointment group was 0.05 ± 0.23
Statistically significant (*p = <.0001) improvement in Skin Burning / Stinging was observed in Psorolin B ointment group when compared at visit 4 Table 7 & Table 8.
| Skin BurPlotning / Stinging | Day 0 (Visit 1) (Baseline) |
Assessment Visit 1 (Visit 2) |
Assessment Visit 2 (Visit 3) |
End of treatment visit (Visit 4) |
|---|---|---|---|---|
| Psorolin B Ointment | ||||
| N | 20 | 20 | 20 | 19 |
| Mean | 1.65 | 0.75 | 0.20 | 0.05 |
| SD | 0.49 | 0.44 | 0.41 | 0.23 |
| Min | 1 | 0 | 0 | 0 |
| Max | 2 | 1 | 1 | 1 |
| Median | 2 | 1 | 0 | 0 |
| CV | 29.66 | 59.23 | 205.20 | 435.89 |
| p Value (Paired T Test) Each visit compared with baseline | <.0001 | <.0001 | <.0001 | |
Table 7. Skin Burning / Stinging with Psorolin B Ointment
| IGA | Day 0 (Visit 1) (Baseline) |
Assessment Visit 1 (Visit 2) |
Assessment Visit 2 (Visit 3) |
End of treatment visit (Visit 4) |
|---|---|---|---|---|
| Psorolin B Ointment | ||||
| N | 20 | 20 | 20 | 20 |
| Mean | 2.70 | 1.85 | 1.25 | 1.00 |
| SD | 0.47 | 0.59 | 0.44 | 0.00 |
| Min | 2 | 1 | 1 | 1 |
| Max | 3 | 3 | 2 | 1 |
| Median | 3 | 2 | 1 | 1 |
| CV | 17.41 | 31.74 | 35.54 | 0.00 |
| p Value (Paired T Test) Each visit compared with baseline | 0.0102 | 0.0086 | <.0001 | |
Table 8. Investigator Global Assessment (IGA) with Psorolin B Ointment
Discussion
Findings of the present clinical trial of Psorolin B ointment in 20 patients with plaque psoriasis over 4-week period has revealed that the Psorolin B ointment has significant therapeutic effect in treating psoriasis by clearing most of the clinical symptoms associated with the psoriasis. Among various clinical forms of psoriasis, plaque psoriasis is relatively more resistant to treatment[10]. Such resistant psoriasis lesion requires keratinolytic preparations besides the preparation having antiinflammatory and keratin proliferation inhibition property. Due to unpredictable clinical cycle of psoriasis where sudden surge of inflammatory manifestation occurs over a relatively calm phase of psoriasis where the lesion is limited to often mild scaling [11].
Therefore, the treatment product must encompass certain additional therapeutic constituents to address the above need but as on date, such therapeutic benefit is achieved through a combination of drugs including steroids [12]. The management of inflammatory stage of psoriasis require prolonged use of steroids or sometime mutagenic drug like Methotrixate and often such preparations would result in serious side effects [13]. Psoriasis being an incurable disease, prolonged medication with such drugs are needed and in due course of time, both the primary medical problem – psoriasis and the side effects due to steroid or mutagenic drugs, would affect the overall health and wellbeing of the patient.
Psorolin B has been studied at the laboratory level extensively on keratinocyte cell line in order to understand how the Siddha drug would reduce the cell proliferation and cell turnover time, modify the release of pro-inflammatory mediators from the cultured cells, modifying effect of Psorolin B in the expression of S100A7 protein that invite an array of inflammatory events, histidine decarboxylase enzyme inactivation, suppressive effect on collagenase, elastase, hyaluronidase, glycation process and anti-oxidant effect by different methods. All laboratory level evaluations clearly reveal the pluripotent, polymorphic therapeutic nature of Psorolin B owing to the multiple herbal ingredients present in the formulation.
The present clinical evaluation was done on 20 patients over 4-week duration with evaluation on certain distinct parameters like TPSS, erythema, induration/thickness, scaling. Other parameters such as dryness, pruritus and burning/stinking were also considered.
At the end of fourth week, all parameters such as total plaque severity score, erythema, induration/thickness and scaling showed remarkable reduction.
TPSS from baseline value of 5.6 to 1.25 on week 12 with percentage reduction of 95.1. The above change was statistically significant with P value <0.0001.
Erythema has improved from baseline value of 1.75 to 0.05 on week 12 with percentage reduction of 97.14. The above change was statistically significant with P value <0.0001.
Induration/thickness has improved from baseline value of 2.10 to 0.95 on week 12 with percentage reduction of 54.76%. The above change was statistically significant with P value <0.0001.
Scaling has improved from baseline value of 1.85 to 0.25 on week 12 with percentage reduction of 86.49%. The above change was statistically significant with P value <0.0001.
Dryness was reduced from baseline value of 1.85 to 0.11 on week 12. The above change was statistically significant with P value <0.0001.
Skin pruritus was reduced from baseline value of 1.85 to 0.84 on week 12. The above change was statistically significant with P value <0.0001.
Skin burning was reduced from baseline value of 1.65 to 0.05 on week 12. The above change was statistically significant with P value <0.0001.
The self-assessment of all the patients also agreed with the clinical findings. Statistical analysis showed the level of reduction of the above parameters to be significant.
The present clinical trial has clearly established the clinical efficacy of Psorolin B ointment in the treatment of Psoriasis. Although the present trial was limited to 20 patients with plaque psoriasis but the findings clearly reveal the clinical efficacy of Psorolin B ointment is scientific. In the light of plethora of scientific study done on Psorolin B ointment in the laboratory, the clinical trial findings can be stretched to other clinical types of Psoriasis as well.
Our recent study on the effect of Psorolin B in modifying S100A7 protein suggest the usefulness of Psorolin B in the management of inflammatory stage of Psoriasis. We, in the light of the clinical trial findings and other data on Psorolin B ointment, state that a management solution to Psoriasis is possible but continuous usage of Psorolin B may be required.
References
- Furue K, Ito T, Tsuji G, Kadono T, Nakahara T, Furue M. Autoimmunity and autoimmune co?morbidities in psoriasis. Immunol. 2018;154(1):21-7.
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475.
- Gendrisch F, Haarhaus B, Krieger N, Quirin KW, Schempp CM, Wölfle U. The effect of herbal medicinal products on psoriasis-like keratinocytes. Biomol. 2021;11(3):371.
- Sundarrajan S, Lulu S, Arumugam M. Deciphering the mechanism of action of wrightia tinctoria for psoriasis based on systems pharmacology approach. J Altern Complement Med. 2017;23(11):866-78.
- Aruna v, Amruthavalli GV And Gayathri R. Psorolin B – A new ‘taxon’ in the treatment of psoriasis and the science of electrochemical homeostasis. World J Pharm Res. 10( 8) 554-559.
- Botanical warriors to combat pro-inflammation mediators – A way forward for Psoriasis management. Amruthavalli G.V*, Aruna.V, Gayathri.R. Int Inven Sci J. 2021;5(9), P.No.21-24.
- Importance of thermo, hydro and lipid chemistry of psorolin b and its treatment significance in psoriasis. Eur J Pharm Med Res. 2021;8(7).
- GV A, Aruna V, Rajagopal G. Psorolin B: a formulation with synchronized, synergistic scooping of botanicals and associated exodus therapeutic benefit to psoriasis. Int J Res. 2022;8(1):85.
- Aruna V, Amruthavalli GV, Gayathri R. ‘Agua-Phobia’a Necessary Prerequisite for Healing Psoriasis: Proof from Psorolin B. J Drug Deliv Ther. 2022;12(6-S):7-9.
- Staubach P, Zimmer S. Plaque psoriasis–more than a skin disorder. Med Monatsschr Pharm. 2017;40(6):231-3.
- Man AM, Or?san MS, Hoteiuc OA, Ol?nescu-Vaida-Voevod MC, Mocan T. Inflammation and Psoriasis: A Comprehensive Review. Int J Mol Sci. 2023;24(22):16095.
- Lebwohl M, Menter A, Koo J, Feldman SR. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50(3):416-30.
- Hamed KM, Dighriri IM, Baomar AF, Alharthy BT, Alenazi FE, Alali GH, Alenazy RH, Alhumaidi NT, Alhulayfi DH, Alotaibi YB, Alhumaidan SS. Overview of methotrexate toxicity: A comprehensive literature review. Cureus. 2022;14(9).
Indexed at, Google Scholar,Cross Ref.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref