- Biomedical Research (2008) Volume 19, Issue 1
An investigation of the value of the Tetrahymena pyriformis as a test organism for assessing the acute toxicity of antidepressants
Uma S.1*, Kelly J.P.2 and Rajasekaran S.K.31Department of Physiology & Endocrinology,Na-tional University of Ireland Galway, Ireland.
2St. Mat-thew’s Medical University, Grand Cayman, Cayman Islands, Department of Pharmacology & Therapeutics,Na-tional University of Ireland Galway, Ireland.
3Department of Pharmacology & Clinical Therapeutics,Na-tional University of Ireland Galway, Ireland.
- *Corresponding Author:
- Uma Senthilkumar
Department of Physiology & Endocrinology
St. Matthew’s Medical University
Grand Cayman
Cayman Islands
e-mail: usenthilkumar@smucayman.com
Accepted date: December 27 2007
Abstract
Antidepressants differ greatly from class to class and within individual members of a class in terms of their adverse drug profile and their toxicity in acute overdose conditions. To re-duce the use of animals, a number of in vitro tests are now under investigation to determine the acute toxicity of drugs. The objective of this study was to investigate fresh water proto-zoa Tetrahymena pyriformis with regard to the acute toxicity of antidepressants. The cyto-toxic effects of a range of currently marketed antidepressants were evaluated using the MTT assay, and the IC50 values were determined for each. These IC50 values did not corre-late with the FTI (Fatal Toxicity Index) values in humans. Thus it can be concluded that the Tetrahymena pyriformis cytotoxicity assay is a poor predictor in evaluating the acute toxicity of antidepressants in humans, which is in keeping with other in vitro methodologies using mammalian cells. It can be concluded that, in the absence of credible in vitro tests, acute tox-icity in intact animals will continue to be the method for predicting the acute toxicity of an-tidepressants.
Key words
Tetrahymena pyriformis, Antidepressants, Alternatives, Acute toxicity, MTT assay Correspondence: usenthilkumar@smucayman.com
Introduction
Most of our current understanding about the toxicity of various chemicals comes from animal data, and as An-drew Rowan notes in his critical evaluation of animal re-search, Of Mice, Models & Men, "there is no doubt that our knowledge of the risks to humans of most chemicals is very inadequate" [1]. In recent years the practice of acute toxicity testing in animals has been criticized by animal right activists and antivivisectionists, as well as moderate organizations, e.g. FRAME (Fund for the Re-placement of Animals in Medical Experimentation). Ser-endipitously, Russell and Burch's proposal of the three R’s, i.e. Replacement, Reduction and Refinement alterna-tives has coincided with the development of molecular biology [2], as well as with vast improvements in tech-nique for such things as propagating cells in culture and measuring molecular processes. However, some form of acute toxicity testing in animals is still deemed to be nec-essary in evaluating a test compound’s safety. Such inves-tigation can provide useful information on signs of intoxi- cation, target organs of toxicity and cause of death. This can be achieved if the experiments are performed by well trained personnel, using all available techniques to moni-tor physical and pathomorphological changes of the or-gans [3]. However, there are many potential advantages of in vitro systems in toxicity testing are numerous. In vitro tests are usually quicker and less expensive. Experimental conditions can be highly controlled and the results are easily quantified. However, the relative simplicity of non whole-animal testing results in limitations as well. Cells or tissues in culture often cannot predict the effect of a toxin on a living organism with its complex interaction of nervous, endocrine, immune, and hematopoietic systems [4]. Although tissue and cell culture assays are currently the most popular in vitro tests for evaluating acute toxic-ity, other techniques are gaining prominence. These tech-niques may involve the use of bacteria and lower life forms for the determination of toxicity [5]. In the past decade, some laboratories have shown a significant corre-lation between in vivo or in vitro toxicity using mammal-ian cells and in vitro test systems using bacteria and pro- tozoa [6], [7]. At present the in vitro methods available for determining sensitizers principally involve the use of cell mono layers, co-cultures or isolated skin explants. There are other fields of research which could lead to the development of more complex test systems. These are able to more accurately mimic the events which lead to skin sensitization in vivo and include structure activity relationships (SAR), quantitative structure activity rela-tionships (QSAR) and expert systems. It is hoped to in-vestigate the immunology and cell biology underlying the phenomenon of skin sensitization, to determine the most realistic opportunities for successful development of non-animal alternatives [8]. Protozoa are the simplest eu-karyotic organisms but despite this contain almost the same metabolic systems as higher animals [9]. In addition they are appropriate organisms for toxicological studies, owing to their ease of culturing, short life cycle and large surface contact with the environment. The most common protozoan model used in toxicological studies is Tetrahy-mena pyriformis [10]. Antidepressants represent a major achievement in the treatment of patients suffering from depression. Unfortunately, reports of lethal overdoses of antidepressants started appearing early in the course of their clinical use, demonstrating their potential for harm as well as their therapeutic effects [11]. Antidepressant drugs are one of the most frequently ingested substances for accidental poisoning or a suicide attempt. The Ameri-can Association of Poison Control Centers reported that antidepressants were the most frequent cause of death from drug ingestion in the years 1983 and 1984 [12].
In the era of growing concerns of animal rights and the human safety issues related to exposure to biological samples, there is a huge need for developing alternative methods of toxicological evaluation of drugs. In this study we made an attempt to investigate the possibility of using Tetrahymena pyriformis as an alternative for studying the drug’s effect on cell proliferation. This study concentrated on one particular in vitro cytotoxicity test using Tetramy-mena pyriformis, and investigating the effects of antide-pressants. MTT (3-(4,5-dimethyethiazol-2-yl)-2,5-diphe-nyltetrazolium bromide) assay was used for measuring cytotoxicity. The tetrazolium salt reduction method is widely used as a means of examining cell proliferation and viability. The internal environment of proliferating cells is more reduced than that of non-proliferating cells [13]. The tetrazolium salts are reduced at sites in the mi-tochondrial electron transport system and are a test for succinate dehydrogenase activity. The reaction converts yellow salts to blue crystals that are dissolved and can be read spectrophotometrically. The most frequently used of these are MTT (3-(4,5-dimethyethiazol-2-yl)-2,5-diphen-yltetrazolium bromide), XTT (sodium 3’-[1-phenyl-amino)-carbonyl]-3,4-tetrazoliuum]-bis (4-methoxy-6-ni-tro) benzene-sulfonic acid hydrate, and MTS. Tetrazolium salt reduction, as an indicator of cell growth, has been used in models for screening cytocidal chemicals [14], [15] or cell growth promoting factors and cytokines [16].
Materials and Methods
All the antidepressant compounds tested were obtained from Sigma Aldrich, Dublin, Ireland. All assays are con-ducted in axenic conditions. 18–24 h cultures of Tetrahy-mena pyriformis used were strain GL, ref. CCAP/1630/1F from Strains of Culture Collection of Algae and Protozoa, Scotland, United Kingdom. The cells were grown to exponential phase at room temperature in Proteose Pep-tone Yeast Extract Medium (PPY) which consisted of 2% w/v protease peptone and 0.25% w/v yeast extract at pH 7.0–7.5. The density of T. pyriformis cultures was ad-justed in fresh PPY in order to obtain 104 cells/ml. The cells were initially counted using a Sedgwick Rafter counting chamber.
A volume of 80 μl of suspended T. pyriformis was added to each well of a 96-well flat-bottom microtitre plate along with 10 μl of the antidepressants which were tested at a range of concentrations. The well plate was then in-cubated at 28oC under 5% CO2 for 1 hr. Then, 10μl of MTT (3-(4,5-dimethyl-tiazol-2-yl)-2,5 diphenyl-tetrazo-lium bromide (Sigma, Chemical Co., Dorset, UK) stock solution (10 mg/ml in distilled water) were added and time 0 readings were taken at 540 nm using the spectro-photometer and the plates were incubated at 280C for 4 hrs. The MTT reduction was stopped with 200 μl of a solution containing 50% dimethylformamide and 10% sodium dodecyl sulfate-(DMF/SDS) without removing the medium. The formazan production due to succinate dehydrogenase activity was measured 30 min after DMF/SDS incubation.
Concentration-response graphs were generated for each antidepressant using GraphPad Prism software. These graphs were analysed using a curve fit for sigmoid dose-response, and IC50 values were derived. Results are ex-pressed as mean IC50 with the 95% confidence intervals.
Results
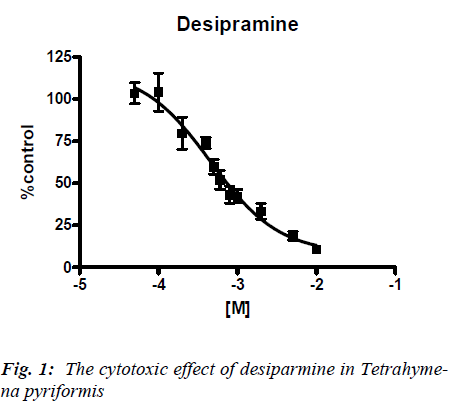
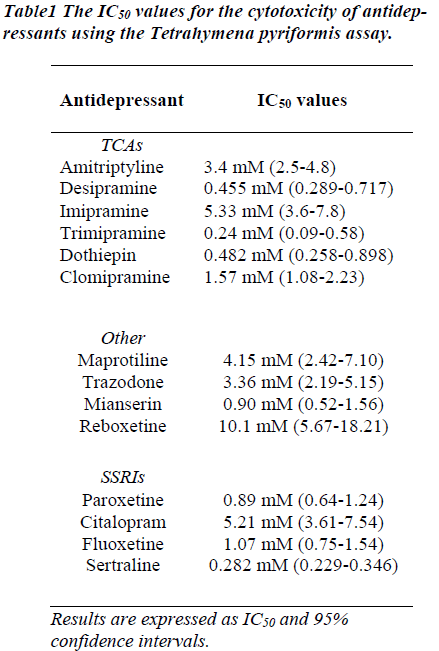
Concentration response curves were obtained for each antidepressant when incubated with Tetrahymena pyri-formis and the IC50 values determined. A typical concen-tration response is illustrated for desipramine (Figure 1). The results for each antidepressant are summarized in Table 1. As can be seen from the table, The IC50 of the tested compounds in Tetrahymena pyriformis ranged from 0.24 mM for trimipramine to 5.33 mM for imipramine using the MTT assay.
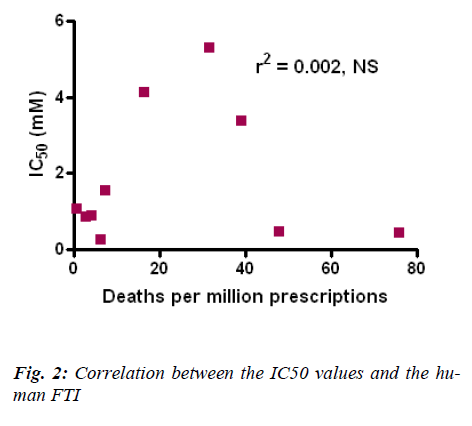
The results obtained in this study using Tetrahymena pyriformis were then compared and correlated with the human Fatal Toxicity Index (FTI) for antidepressants (Fig. 2). FTI data was taken from Henry et al [17]. The above analysis shows that there is a very poor correlation between the human FTI and the IC50 values obtained for the antidepressants using Tetrahymena pyriformis.
FTI data updated from Henry et al [17].
Discussion
The acute IC50 of the tested compounds in Tetrahymena pyriformis ranged from 0.24 mM for trimipramine to 5.33 mM for imipramine using the MTT assay. The general trend in the results obtained shows that, on average, the heterocyclic compounds were determined to have higher IC50 values than the TCA and SSRI compounds, which by inference would make the heterocyclic compounds more toxic to cells. This was a surprising result considering the general consensus that heterocyclic compounds are safer than TCA drugs [18].
Use of human blood cells in the cell proliferation studies is limited because of the safety concerns, vaccination, availability of trained professional to draw blood, com-pensation for the donor, transport of blood samples to the lab and the handling of blood samples in the lab etc. The use of animal blood cells for such studies is again limited by many of the above said factors plus the ethical issue of animal sacrifice. The least one can do in basic research is to avoid tests which cause severe suffering to animals, as is required in Switzerland and other European countries by binding ethical principles and guidelines. The increas-ing standard of approval and control procedures has im-proved the situation over the years. There are many ex-amples of successful alternative methods in basic re-search. But, the application of such methods is in most cases limited to the laboratories in which they were de-veloped, calling for technology transfer [19].
Tetrahymena pyriformis has been used extensively in the past to perform various toxicological assays and has been consistent in reproducing the results [20]. Hence this or-ganism is being commonly used as a toxicological tool.
The data obtained from this MTT assay shows a good linear regression with the data obtained from assays using rat blood PBMC’s, human neutrophils and rat kidney cells [21], [22], [23]. The FTI and the rat LD50 do not however correlate with the results obtained from Tetrahymena pyriformis.
It can be concluded from the results and analysis that Tet-rahymena pyriformis can be considered to perform acute toxicity studies involving antidepressants. MTT assay seems to be a viable test for studies with Tetrahymena pyriformis. The human FTI and the IC50 values obtained from this study does not correlate with each other. Its ability to produce toxicity data relevant to human remains questionable. Further studies are warranted to fully estab-lish the role and use of Tetrahymena pyriformis in acute toxicity studies.
References
- Rowan A. Shortcomings of LD50-values and acute tox-icity testing in animals. Acta Pharmacol Toxicol (Co-penh) 1983; 52: 52-64.
- Russell, WMS, Burch RL. The principles of humane experimental technique.1969; Methuen & Co., Ltd., London.
- Flury-Roversi M. Significance of the LD test. Arch. Toxico 1981; 47: 77-99. 50
- Eisenbrand G, Pool-Zobel B, Baker V, Balls M, Blaau-boer BJ, Boobis A, Carere A, Kevekordes S, Lhugue-not JC, Pieters R, Kleiner J. Methods of in vitro toxico-logy: Food and chemical technology 2002; 40: 193-236.
- Flint OP. Predicting In Vivo ToxicityToxicology in Vitro, 1998; 12: 591-595
- Bruner LH, Kain, Dl, Roberts DA, Parker RD. Evalua-tion of seven in vitro alternatives for ocular safety test-ing. Fundam Appl Toxicol 1991; 17: 136-149.
- Calleja MC, Persoone G, Geladi P. Human acute toxic-ity prediction of the first 50 MEIC chemicals by a bat-tery of ecotoxicological tests and physicochemical properties. Food Chem Toxicol 1994; 32: 173-87.
- Devillers J. Prediction of mammalian toxicity of or-ganophosphorus pesticides from QSTR modeling. SAR QSAR Environ Res 2004; 15: 501-510
- Hauser C. Cultured epidermal Langerhans cells activate effector cells for contact sensitivity. J Invest Dermatol 1990; 95: 436-440
- Nalecz-Jawecki G, Rudz B, Sawicki J. Evaluation of toxicity of medical devices using Spirotox and Micro-tox tests. J of Biomed Mat Res 1997; 35: 101-105.
- Sauvant MP, Pepin D, Piccinni E. Tetrahymena pyri-formis: a tool for toxicological studies. Chemosphere, 1999; 38: 1631-1669.
- Molcho MD, Stanley M. Antidepressants and suicide risk: Issues of chemical and behavioural toxicity. Jour-nal of Clin. Psychopharmacol 1992; 12: 13s-19s.
- Litovitz T, Veltri JC. Annual report of the American Association of Poison Control Centres national data collection system. Am J Emerg Med 1985; 3: 423-450.
- Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunnol. Meth 1983; 65: 55-63
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czer-winski DL, Fine DL, Abbot BJ, Mayo JG, Shoemaker RH, Boyd MR Feasibility of drug screening with pan-els of human tumour cell lines using a microculture tetrazolium assay. Cancer Res 1998; 48: 589-601.
- Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/ formazan as-say for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988; 48: 427-433.
- Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Stober W. editors. Measurement of tumour necro-sis factor α and β. Unit 6.10. In: Current Protocols in Immunology. John Wiley and Sons, In 1996.
- Henry JA, Alexander CA. Sener EK. : Relative mortal-ity from overdose of antidepressants. BMJ. 1955; 310: 221-224
- Katzung G. Basic and Clinical Pharmacology, 9th edi-tion, Lange publication 2004
- Gruber FP, Hartung T. Alternatives to animal experi-mentation in basic research. ALTEX. 2004; 21: 3-31
- Seward JR, Sinks GD, Schultz TW. Reproducibility of toxicity across mode of toxic action in the Tetrahymena population growth impairment assay. Aquat Toxicol 2001; 53: 33-47
- Darcy Philip. An evaluation of methods for the assess-ment of the acute toxicity of antidepressants. M.Sc, Department of Pharmacology and Therapeutics, Trinity College, Dublin 1998.
- Galvin M. The cytotoxicity profile of antidepressants in human neutrophils. M.Sc, Department of Pharmacol-ogy, National University of Ireland Galway 2003.
- Henneghan A. Determination of the cytotoxicity profile of antodepressants in rat spleen PBMC’s using rezaurin reduction assay. B.Sc., Department of Pharmacology, National University of Ireland Galway 2004.