Research Article - Biomedical Research (2016) Volume 27, Issue 4
An impedimetric comparison of cytotoxicity caused by cisplatin and doxycycline on osteosarcoma cell lines
Yong-Xiang Shi1, Cheng-Zhen Liang1, Wei-Liang Shen1, Yu-Xiang Xiao1, Qi-Xin Chen1, Hao Peng2 and Wei-Shan Chen1*1Department of Orthopedic Surgery, 2nd Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310009, PR China
2Department of Orthopedics, Renmin Hospital of Wuhan University, Wuhan 430060, PR China
- *Corresponding Author:
- Wei-shan Chen
Department of Orthopedic Surgery
2nd Affiliated Hospital, School of Medicine
Zhejiang University
88 Jie Fang Road
Hangzhou 310009
PR China
Accepted date: April 07, 2016
Abstract
The present paper describes a comparative characterization of cytotoxic effects of doxycycline and cisplatin on osteosarcoma cells by using impedance sensing techniques. The impedance sensing device was obtained from Applied Physics and the osteosarcoma cells were cultured on the electrodes of the purchased devices. The impedimetric cytotoxic natures of both the drugs on cells are investigated by using impedance analyzer at 10 mV in a frequency range of 100 Hz to 1 MHz. Impedance spectrum decreases drastically in drug treated samples above 10 μM dose as cell death and detachment from the electrode surfaces increases rapidly. However the cisplatin shows better results over doxycycline as observed from impedimetric as well as conventional results. The cisplatin treated sample shows lesser impedance than the doxycycline treated sample as the IC50 value of doxycycline is more than cisplatin on osteosarcoma cells.
Keywords
Bioimpedance, Cisplatin, Doxycycine, Osteosarcoma, Cytotoxicity.
Introduction
Osteosarcoma is an aggressive malignant tumor that arises in bone, in which the malignant cells osteoblastic differentiation and produce osteoid [1]. Osteosarcoma occurs most frequently in adolescents at the age of 10 to 20 years while secondary osteosarcoma predominates in adult patients [2]. It is regarded as the most common sarcoma in bone, but it is quite rare in appearance. Osteosarcomas represent less than 1% of cancers overall and the 5-year survival rate after amputation is only 5– 20% [3]. At all ages males are more prone to this disease than female patients. This cancer occurs in humerus, femur, fibula, vertebrae, tibia, and ilium with a greatest case in femur and tibia [4,5]. The pathogenic factors such as genetic impairments, viral infection, chemical reagents, and radiation are responsible to form this type of cancer [4-6] Osteosarcoma is regularly treated with combined therapies such as surgery, chemotherapy and radiation therapy [7].
Regarding chemotherapy the drugs such as cisplatin and doxycycline are more often used to treat the osteosarcoma and the conventional methods such as MTT assay and imaging are used to assess the cytotoxicity of the used drugs. But such conventional methods are time consuming and expensive as well as cell dies with the end of the experiment. Thus a method is required to study the live cell behaviours and in the present day electric cell substrate impedance sensing (ECIS) is used to study the cellular properties in a culture.
ECIS is a noninvasive biophysical technique which is used to study the different aspects of cellular characterizations such as cell growth [8-10] and cell death [11-15]. However, there is no report found assessing the cytotoxic effect of cisplatin and doxycycline by ECIS technique. This paper presents comparative impedimetric assessments of the cytotoxic effect of cisplatin and doxycycline on osteosarcoma cells by using ECIS devices.
Materials and Methods
Materials and reagents
8W10E ECIS devices were supplied by Applied Biophysics, USA. Doxycycline and cisplatin were purchased from Sigma (China). RPMI 1640, streptomycin (10000 μg/mL), penicillin (10000 U/mL), L-glutamine (200 mM), fetal bovine serum (FBS), were purchased from Invitrogen (China). All other chemicals used in the present experiments were purchased from Invitrogen (China).
Cell culture
The human osteosarcoma cell such as U2OS was supplied by American Type Culture Collection and maintained with RPMI 1640 medium. Then the cells were cultured in RPMI 1640 supplemented with essential antibiotics and growth factors such as FBS, penicillin, streptomycin, and glutamine (pH 7.3) with appropriate concentrations. The cell number was adjusted to 4 × 105/mL. The osteosarcoma cells were cultured in impedance sensing devices and 96-well plates on by using RPMI 1640.
Impedance measurement
The ECIS device possesses eight wells and each well contains ten gold microelectrodes (250 μm diameter). The microelectrodes were used to culture the cells on their surfaces and help to measure the current flow passing through the medium. The dimension of the culture area is 0.8 cm2 and the volume of the well-used for culture is 600 μl. The impedance sensing device was kept in a tissue culture incubator (37°C and 5% CO2) for cell culture and measuring of cell impedances. The array plates were equilibrated with 300 μl of RPMI 1640 for 12 h and subsequently 1 × 105 cells were cultured to each well in 300 μl media.
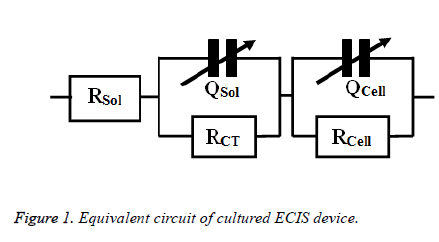
The toxic effects of doxycycline and cisplatin were investigated by obtaining the impedance of drug treated osteosarcoma cells. The impedance is measured in a frequency range of 100 Hz to 1 MHz in a logarithmic scale after 24 h of drug treatment. The impedance were measured ten times repeatedly and averaged to get man impedance for each frequency. This mean value of impedance obtained from the experiment was imported to Zsimp Win (Version 3.10) for fitting purposes the equivalent circuit used here was obtained from an old literature [11] and was illustrated in Figure 1.
Experimental protocols
Before the cell seeding, the impedance sensing devices was disinfected with 75% ethanol for 20 min, dried with nitrogen, and irradiated with ultraviolet radiation for 20 min. MEM (300 μl,) was then added to the wells of the device and maintained at 37°C for 30 min. Next, the osteosarcoma cell suspensions (300 μl, 1 × 105 cells) were added into each well. Then the device was place in an incubator to facilitate the growth of cells on electrode surfaces and subsequent measurement of impedance. The doses of doxycycline and cisplatin (0, 5, 10, and 15 μM) were added into different wells of the impedance sensing device after 30 min of inoculation to investigate the cytototoxic effects of the drugs.
Comparison of impedance data with MTT assay
Osteosarcoma cells (1x104 cells/well) were cultured and allowed to grow for 24 h and treated with doxycycline and cisplatin at varying concentrations (10 nM to 10 μM) for 24 h. IC50 value was measured by MTT dye reduction assay as described in the previous literature [16].
Trypan-blue exclusion assay
Osteosarcoma cells (1x104 cells/well) were cultured in 96-well plates and drug doses such as 0, 5, 10, and 15 μM is added to different wells and treated for 24 h. Then cells were rinsed with phosphate buffered saline, treated with 0.25% of trypsin and stained with 0.4% trypan-blue. Then the cell viability was counted by using automated cell counter (Biorad).
Comparison of impedance data with phase contrast images
Osteosarcoma cells were plated on coverslips in RPMI 1640 medium. The cells were treated with varying concentration (0, 5, 10, 15 μM) of doxycycline and cisplatin for 24 h. Phase contrast images of drug treated cells were taken for the treated cells after 24 h of culture by using phase contrast microscopy.
Results
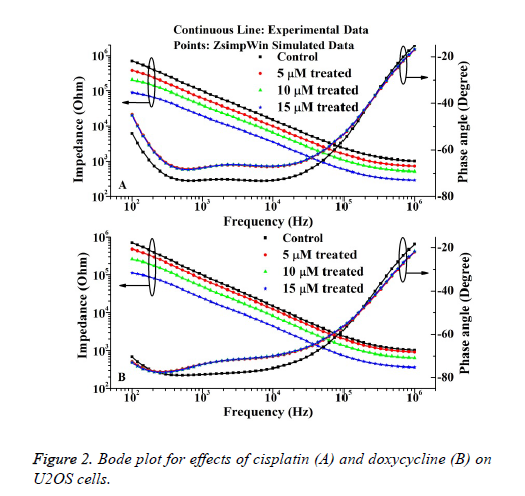
The bode plots for the cytototoxic effects of cisplatin and doxycycline on osteosarcoma cells after 24 h are described in Figure 2. The investigational data are fitted quite well with the used equivalent circuit as shown in Figure 1. This result can be compared to the previous result regarding cell culture on electrode surfaces [17].
It is evident from the Figure 2 that the impedance decreases steadily with increase of frequency. However, the cisplatin shows more cytotoxic effect than the doxycycline as observed from the Figure 2. The relative standard deviations (RSD) for control and treated experiments are stayed below 10% justify the reproducibility nature of the investigation.
The IC50 value of doxycycline and cisplatin obtained from MTT assay is 8.55 ± 0.3243 μΜ and 6.23 ± 0.5621 μΜ. The percentages of living cells for control and doxycycline treated samples (5 μM, 10 μM and 15 μM) are 97 ± 3, 75 ± 4, 49 ± 3, 31 ± 5 respectively. Also the percentage of living cells for cisplatin treated samples are 63 ± 3, 40 ± 5, 25 ± 4 respectively The results obtained from the MTT assays correlate well with the impedance data as represented in Figure 2. The cell viability (%) was obtained from the impedance by using the following equation (1) extracted from the previous literature [11].
Where, ZC and ZT represent the mean value of impedance for untreated and treated sample at 24 h respectively. The IC50 values of doxycycline and cisplatin estimated from the impedance data are 8.61 ± 0.5673 μΜ and 6.19 ± 0.3214 μΜ respectively. Thus it is evident that, the IC50 value obtained by impedimetric method correlate well with the values as obtained by MTT assay method.
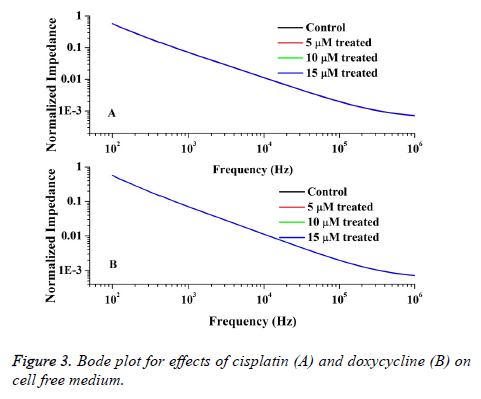
Also the impedimetric role of drugs on RPMI 1640 is measured by using medium with different doses of drugs and the Bode plot is depicted in Figure 3. From the figure it is obvious that the spectra of impedance values for different drug doses of doxorubicin and cisplatin do not fluctuate significantly from the control data with 0.004 % error. Therefore it can be understood that the drugs in medium do not interfere with the cell impedance measured in tissue culture.
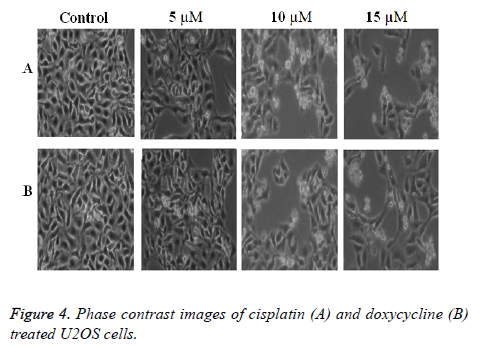
The phase contrast images as shown in Figure 4 indicate that the cell death increases with the increase of drug doses in both the cases. This arises due to dose dependent cell death in culture. However in the present experiment cisplatin shows better result than the doxycycline as evident from the Figure 4. Also the conventional data such as MTT assay results and microscopic images correlate well with the impedance data of cytotoxicity.
Discussions
The decrease in impedance arises as drugs interact with cells resulting cell death and detachment from the electrode surfaces. With the increase of drug doses the impedance decreases in both the cases showing the dose dependent nature of cisplatin and doxycycline. The cell death is prominent in the treated sample over 10 μM in both the cases leading to decline in the spectrum of impedance compared to control sample as described in Figure 2. The present results can be compared to the decreasing impedance with the increasing drug doses of different chemotherapeutic agents as described in previous literatures [13,18,19]. Also the cell death shown by phase contrast images as described in Figure 4 can be comparable to the impedance results as well as the results described in the previous literatures [20-22]. The mechanism of the ECIS sensor depends on the attachment and detachment of the cells from the electrode surfaces and the mechanism of the sensor can be compared to the previous result [13]. When the drugs act on the cultured cells, the cell dies and detaches from the electrode surfaces. Thus the impedance decreases due less availability of live cells on electrode area. Both the drugs show apoptotic nature in a dose dependent manner. However cisplatin shows better cell death than the doxycycline as observed from the impedimetric as well as conventional results. The cytotoxic nature of drugs should be explored further to understand the mechanism involved in better cell death in case of cisplatin than doxycycline.
Acknowledgements
The authors received financial support form 2016 Zhejiang Medicines Health Science and Technology Program General Studies Program (No: 2016134432) and 2016 Zhejiang Provincial Natural Science Foundation of Youth Project (NO: LQ16H060001).
References
- Anderson ME. Update on Survival in Osteosarcoma. Ortho Clin North Am 2016; 47: 283-92.
- Shao J, Wang L, Zhong C, Qi R and Li Y. AHSA1 regulate proliferation, apoptosis, migration, and invasion of osteosarcoma. Biomed Pharmacother 2016; 77: 45-51.
- Ottaviani G, Jaffe N. The Epidemiology of Osteosarcoma. In: Jaffe N, Bruland OS and Bielack S, (eds.) Pediatric and Adolescent Osteosarcoma. Springer US, 2010; 3-13.
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009; 115: 1531-1543.
- Ottaviani G, Jaffe N. The Etiology of Osteosarcoma. In: Jaffe N, Bruland OS and Bielack S, (eds.). Pediatric and Adolescent Osteosarcoma. Springer US, 2010; 15-32.
- Fuchs B, Pritchard DJ. Etiology of Osteosarcoma. Clin Orthop Rel Res 2002; 397: 40-52.
- Ferguson WS, Goorin AM. Current treatment of osteosarcoma. Cancer Invest 2001; 19: 292-315.
- Su K, Zhou J, Zou L. Integrated multifunctional cell-based biosensor system for monitoring extracellular acidification and cellular growth. Sens Actuators A: Phy 2014; 220: 144-52.
- Wegener J, Keese CR, Giaever I. Electric Cell–Substrate Impedance Sensing (ECIS) as a Noninvasive Means to Monitor the Kinetics of Cell Spreading to Artificial Surfaces. Exp Cell Res 2000; 259: 158-66.
- Xiaoqiu H, Nguyen D, Greve DW, Domach MM. Simulation of microelectrode impedance changes due to cell growth. IEEE Sens J 2004; 4: 576-83.
- Pradhan R, Mandal M, Mitra A, Das S. Monitoring cellular activities of cancer cells using impedance sensing devices. Sens Actuators B: Chem 2014; 193: 478-83.
- Pradhan R, Mandal M, Mitra A, Das S. Assessing Cytotoxic Effect of ZD6474 on MDA-MB-468 Cells Using Cell Based Sensor. IEEE Sens J 2014; 14: 1476-81.
- Pradhan R, Rajput S, Mandal M, Mitra A, Das S. Frequency Dependent Impedimetric Cytotoxic Evaluation of Anticancer Drug on Breast Cancer Cell. Biosens Bioelectron 2014; 55: 44-50.
- Pradhan R, Rajput S, Mandal M, Mitra A, Das S. Electric cell-substrate impedance sensing technique to monitor cellular behaviours of cancer cells. RSC Adv 2014; 4: 9432-8.
- Müller J, Thirion C, Pfaffl MW. Electric cell-substrate impedance sensing (ECIS) based real-time measurement of titer dependent cytotoxicity induced by adenoviral vectors in an IPI-2I cell culture model. Biosens Bioelectron 2011; 26: 2000-5.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55-63.
- Pradhan R, Das L, Chatterjee J, Mitra MMA, Das S. Monopolar impedance sensing microdevices for characterization of cells and tissue culture. Sens Lett 2013; 11: 466-75.
- Eldawud R, Stueckle TA, Manivannan S. Real-time analysis of the effects of toxic, therapeutic and sub-therapeutic concentrations of digitoxin on lung cancer cells. Biosens Bioelectron 2014; 59: 192-9.
- Kustermann S, Boess F, Buness A. A label-free, impedance-based real time assay to identify drug-induced toxicities and differentiate cytostatic from cytotoxic effects. Toxicol in Vitro 2013; 27: 1589-95.
- Al-Mohanna MA, Manogaran PS, Al-Mukhalafi Z, Al-Hussein AK, Aboussekhra A. The tumor suppressor p16INK4a gene is a regulator of apoptosis induced by ultraviolet light and cisplatin. Oncogene. 2004; 23: 201-212.
- Furuya K, Ozaki T, Hanamoto T. Stabilization of p73 by Nuclear IκB Kinase-α Mediates Cisplatin-induced Apoptosis. J Biol Chem 2007; 282: 18365-18378.
- Fife RS, Rougraff BT, Proctor C, Sledge Jr GW. Inhibition of proliferation and induction of apoptosis by doxycycline in cultured human osteosarcoma cells. J Lab Clin Med 1997; 130: 530-534.