Research Article - Allied Journal of Clinical Pathology Research (2017) Allied Journal of Clinical Pathology Research (Special Issue 1-2017)
An European multicentric study of esophageal replacements: gastric pullup, jejunal interposition, colonic interposition.
Molinaro F*1, Angotti R1, Bindi E1, Pellegrino C1, Casini L1, Messina M1, Sica M1, Ravasse P2, Petit T2, Chiesa PL3, Lisi G3, Leva E4, Riccipetitoni G5, Noviello C6, Cobellis G6, Martino A6, Casadio G7, Rossi CD7 and Bianchi A8
1Department of Medical Sciences, University of Siena, Italy
2Department of Pediatric Surgery, University of Caen Hospital, France
3Department of Pediatric Surgery, University of Chieti-Pescara, Italy
4Department of Pediatric Surgery, Fondazione IRCCS Ca' Granda-Ospedale Maggiore Policlinico, Milan, Italy
5Department of Pediatric Surgery, "V. Buzzi" Children Hospital, Milano, Italy
6Department of Pediatric Surgery, Università Politecnica delle Marche , Italy
7Department of Pediatric Surgery, Maggiore University Hospital of Parma, Parma, Italy
8Department of Neonatal Surgery, Royal Manchester Children's University Hospital, United Kingdom
- *Corresponding Author:
- Francesco Molinaro
Department of Pediatric Surgery
University of Siena, Italy
E-mail: fmolidoc@me.com
Accepted date: July 14, 2017
Citation: Molinaro F, Angotti R, Bindi E, et al. An European multicentric study of esophageal replacements: gastric pull-up, jejunal Allied J Clinicl Path. 2017 Volume 1 Issue 1 interposition, colonic interposition. Allied J Clinicl Path. 2017;1(1):9-12.
Abstract
Introduction: There are two principals indication to the esophageal replacement: the Long-Gap esophageal atresia and the caustic stenosis. The “main goal” for every surgeon is to choose the best substitute of it. Materials and methods: It is a multicentric retrospective study that includes 27 patients who had undergone to an esophageal replacement between 1990 and 2015. The aim is to find the best esophageal substitute analyzing the experiences of some European hospital Results and patients: Of the 27 patients, 19 (70, 4%) had a long-gap esophageal atresia and 8 (29,6%) a caustic stenosis. 8 patients have had a gastric pull-up, 9 a jejunal interposition and 10 a colonic interposition. The median age at the surgery was 41,3 months. The median follow up was of 72,1 months. We have had a 63% of postoperative complications and the mortality rate was 3.7%. Discussion and conclusion: There are not significantly difference between the three groups regarding the follow up whereas the colonic interposition group has shown a higher rate of postoperative complication and mortality. We cannot find the best choice as esophageal substitute but our study shows that the jejunal interposition could be chosen as first option.
Introduction
The fundamental axiom of the esophageal surgery is that “the best esophagus is the native esophagus”, nonetheless there might be conditions (i.e. the Long-Gap Esophagel Atresia, LGEA) where the choice to preserve the native esophagus embeds several threats for the patient [1]. As a consequence, the esophageal replacement (ER) becomes mandatory. There are many surgical techniques for such replacement, for example: the gastric pull-up (GPU), the gastric tube, the colonic interposition (CI) and the jejunal interposition (JI). However, there is a lack of general consensus regarding the surgery leading to the best results for the patient. Hence, this paper aims at providing a detailed overview of our results about different ERs, including CI, JI and the GPU.
Materials and Methods
A retrospective multi-centric European study, coordinated by the Department of Pediatric Surgery of Siena, has been performed on a sample of 27 patients undertaking esophageal replacements (8 GPU, 9JI, 10 CI) from 1990 to 2015. The analysis carried out has considered cases of department of Paediatric Surgery CHU (Caen), Department of Paediatric Surgery of Parma, Ancona, Milano and Siena.
The need for such replacements related either to Caustic Stenosis (CS) either to LGEA, where an immediate primary end-to-end anastomosis, owing to the distance between the proximal and distal esophageal remnant is usually not practicable. In structuring the analysis we have used the following parameters: mortality, postoperative complications, need for a second operation and the presence of dysphagia achievement of the 50º percentile of the height-weight curves as the last control of our group (subdivided by operation type). The presence of dysphagia was based on the revised Rome III diagnostic criteria. To confirm the soundness of our results we have performed the T Student test and the Fisher’s exact test, considering significant a P value<0.05.
Surgical Techniques
Gastric pull-up
This technique have popularized by Spitz but it was first reported by Sweet at Mass General Hospital in Boston was firstly introduced by Spitz et al. was used [2-7]. The stomach is mobilized through a transverse laparotomy. The right gastric artery is identified and preserved, whereas the left gastric artery is divided close to the stomach. Then, the distal esophagus is dissected and the stomach is mobilised via a posterior mediastinal tunnel into the neck. An esophagogastric anastomosis is done at the apex of the stomach. Many surgeons performed a pyloromyotomy.
Colonic interposition
This technique had the largest published cohort by Hamza et al. was used [8]. The left colon was used for the replacement. First of all, the colonic vascularisation is evaluated through a transverse laparotomy, and the left colonic artery is preserved. The route of the transposition stands behind the stomach and the pancreas. Three anastomosis are made, the first between esophagus and colon, the second between colon, stomach, and the third from where the graft come. A gastrostomy is done. Many surgeons performed a pyloromyotomy and a fundoplication
Jejunal interposition
The technique was introduced was used [9-11]. A control of the bowel and the vascular anatomy is performed from a median laparotomy. The fundus is separated from the diaphragm and the short vessels are severed. The posterior hiatus is opened after the mobilization of the left crus. After the pedicle graft is created: the segment of jejunum close to the Treitz is selected. The first two mesenterical arteries are divided to preserve the peripheral vascularisation. The jejunum is then skeletonized upwards leaving the uppermost part in place for interposition. The anastomosis to restore the bowel continuity is performed and then the graft is mobilised into the neck through the posterior hiatus. Finally, the anastomosis between upper esophagus and jejunum and between jejunum and stomach are made.
Results and Patients
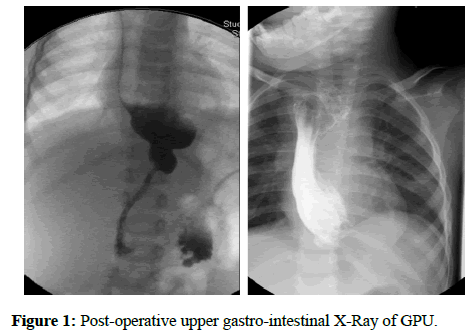
Our sample included 27 patients: 10 females and 17 males (respectively 37% and 63%). In 19 patients the ER was due to LGEA, while for the remaining 8 it was caused by a CS. Before the operation, patients with LGEA undertook an upper gastro-intestinal X-Ray with barium, whereas patients affected by CS had an upper gastro-intestinal X-Ray (Figure 1) with barium and an endoscopy. Patients have been additionally subdivided in three groups: 8 for GPU, 9 for JI and 10 for CI (29.7%; 33.3%; 37%).
All GPUs were done by a posterior mediastinal route and an additional thoracic incision was done in 3 cases. All the 9 JI patients required a single stage operation with an isoperistaltic graft by a posterior mediastinl way. The CI patients needed an isoperistaltic graft by a posterior mediastinal way. 23 patients (85.2%) required a pyloromyotomy. At the end of the surgery, 8 patients got a gastrostomy and 5 received a jejunostomy.
On average, we have observed different ages between the two groups based on the indications to the surgery (ERs due to LGEA and to CS). Precisely, 17.3 months (0.1-72 m) for the first and 80 months (12-192 m) for the second one. We used a nasogastric tube and a drainage chest tube for all those patients ranging between 7 and 9 days after the surgery. They have started feeding after 7 or 8 days from the operation and all of them undertook an antacid therapy.
After the surgery, 17 patients (63%) showed complications, out of which 10 (37%) were early complications and 7 (25.9%) were late complications (Table 1).
| Early complications | Number (%) | Late complications | Number (%) | |
|---|---|---|---|---|
| Colonic Interposition | Claude Bernard Horner Syndrome | 1 (3.7%) | Martial deficiency | 1 (3.7%) |
| Pneumothorax | Occlusive syndrome* | 2 (7.4%) | ||
| Vocal cord paralysis | 1 (3.7%) | Dumping Syndrome | 1 (3.7%) | |
| 2 (7.4%) | Incisional hernia | 1 (3.7%) | ||
| Jejunal interposition | Vocal cord paralysis | 1 (3.7%) | Anastomotic stenosis | 1 (3.7%) |
| Anastomotic leakage | 2 (7.4%) | |||
| Gastric pull-up | Salivary fistula | 2 (7.4%) | Anastomotic stenosis | 1 (3.7%) |
| Jejunal perforation | 1 ( 3.7%) |
Table 1: Patients’ surgery complication, *we have considered only the occlusive syndrome that has treated with another surgery.
Results have been divided by operation: 9 complications for CI (33.3%), 4 for JI (14.8%) and 4 for GPU (14.8%).
We have analysed these differences using the T Student distribution test without finding statistically significant differences (CI p=0.173; JI p=0.4007; GPU p=0.5909). In our study, we have not considered reflux and redundancy as complications since not all the patients undertook an endoscopy or a pH-metre after the surgery and the redundancy was not always symptomatic.
During our study, only 1 patient died; she belonged to the CI group due to an anastomotic leakage complicated by a mediastinitis after 14 days from the surgery. The observed mortality accounted for 3.7%, hence not representing a statistically significant difference (p=1.000). Such mortality value regarding the CI (10%), higher than the one observed in Literature was due to the sample relatively small dimensions [12].
An additional surgery has been required in 3 (11.1%) patients, all of these belong to the CI group. In two cases the second surgery was due to an occlusive syndrome, in one patient the conduit had necrosis. The comparison between techniques had no statistically significant differences (CI/GPU p=0.2157, CI/JI p=0.2105).
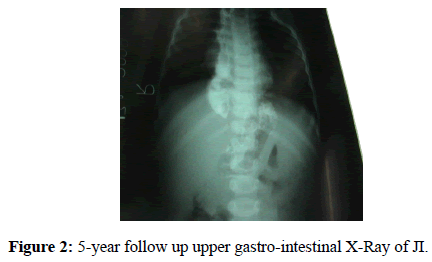
The 72.1 months follow up regarded 23 patients (1 patient died and we lost 3 patients after the discharge). It has lasted for long than 5 years, the maximum required delay to register a significant improvement in the quality of life [13]. In the last follow up only 23 patients (85.2%) did not show problems in feeding and only 14.8% of them presented dysphagia (we supposed that 1 patient was affected by a münchhausen syndrome). Only the JI group lacked patients with dysphagia (2CI, 2GPU) (Figure 2). Although some differences between these groups have arisen, they were not statistically significant (CI/JI p=0737; CI/GPU p=1000; JI/GPU p=0157).
All patients have increased their weight during the follow up, out of which 17 fell within the 50° percentile of the heightweight curves (5 for GPU, 5 for CI and 7 for JI), 9 in the 3° and only 1 below the 3°. We have considered the 50° percentile as a benchmark for a good improvement in height and weight, and even in this case the observed differences were not statistically significant (CI p=0.3029; GPU p=0.9754; JI p=0.2771) (Table 2).
| Postoperative complications (%*) | Mortality (%*) | Second surgery (%*) | Dysphagia (%*) | Achievement of 50° centile (%*) | |
|---|---|---|---|---|---|
| CI | 9 (33.3%) | 1 (3.7%) | 3 (11.1%) | 2 (7.4%) | 5 (18.5%) |
| JI | 4 (14.8%) | 0 | 0 | 0 | 7 (25.9%) |
| GPU | 4 (14.8%) | 0 | 0 | 2 (7.4%) | 5 (18.5%) |
Table 2: Summary of our results, * the percentages are considered on the totality of patients.
Discussion
For the purpose of our study, it is worth mentioning that conserving the native esophagus is not always the best option and the ER might become mandatory. Nevertheless, the best replacement technique still needs to be found. Our retrospective analysis has allowed us to compare three technical surgeries for the ERs, with a particular focus on surgery complications and the patients’ follow up. All the surgeries taken into account have been done by one-step even if sometimes surgeons prefer two-steps surgeries [14,15]. The leakage and the stenosis of the anastomosis are the most analysed by the Literature.
Throughout the study we have described 2 leakages of anastomosis, treated without a second surgery and 1 stenosis of anastomosis. The GPU usually has many advantages, such as the need for a single anastomosis on the gastric fundus with a low rate of leakage and stenosis, a good vascular supply and a good length of the graft. Moreover, there is a low risk of conduit's necrosis. On the other hand, disadvantages include the reflux and the reduction of the mediastinal space as a consequence of the graft size. On the other hand, disadvantages include the reflux and the reduction of the mediastinal space as a consequence of the graft size.
The analysis of the GPU group has shown that this specific technique might be a good way to create the new esophagus [16]. Moreover, it is easy to implement presenting a low complication rate as well, both during and after the surgery. Insights suggested that follow ups have lasted on average 5 years, providing evidences that patients have steadily improved their height and weight, feeding properly after the surgery.
As regards the JI, the advantages relate to the fact that it is the most similar esophagus substitute due to the maintenance of the peristalsis [17] and to the right size of the graft. However, it leads to both, a poor vascularisation of the conduit and the difficulty to create a cervical anastomosis without tension. For the last reason the JI seems to have a higher rate of anastomotic complications [18].
The analysis of the JI group has highlighted a lack of complications during the surgery. In addition, our patients have not been affected by the conduit necrosis, the major complication for this particular surgery. These patients’ follow up has accounted for, on average, 7.3 years, without difficulties in feeding. As a matter of fact, 7 of them have reached the 50° percentile of the height-weight curves. As far as the CI is concerned, the advantages are: the optimal vascularisation and the proper size of the graft. Instead, the disadvantages are: the risk of the conduit ulceration caused by the reflux, the redundancy, the need for three anastomosis and the growth retardation [19,20].
The CI group showed the highest complication rate, including the conduit necrosis as well. Here, we have observed a standalone case of mortality. In addition, during the follow up 5 patients have achieved the 50° percentile of the height-weight curves. Despite its complication rate, the CI is the most widespread technique [15-20].
Conclusion
Our study has provided evidences that the best substitute for an esophagus is likely to be the native one and among the possible substitutes there is not a widespread best practice. In our opinion, all the surgeries evaluated in this study might be the “best choice” for the patient. Therefore, the surgeon’s preferences and abilities become the main drivers when discriminating among different alternatives. Such thesis is confirmed by the lack of statistically significant differences between the GPU, the CI and the JI. However, our results have shown that the JI had a low rate of complications and mortality; this group did not need a second surgery and during the follow up no one has been affected by dysphagia. Moreover, this group presented the higher rate of patients achieving the 50° centile of the height-weight curves. According to these clinical results, we believe that the JI should be the first choice for this surgery in the future.
References
- Rudler JC, Monod-Broca P. “Un cas d’esophagoplastie palliative retro-sternale avec d’ileocolon droit”. Mem Acad Chir. 1951;77:747.
- Spitz L. Gastric transposition for esophageal substitution in children. J Pediatr Surg. 1992;27:252-7.
- Spitz L. Gastric transposition via the mediastinal route for infants with long-gap esophageal atresia. J pediatr Surg. 1984;19:149-54.
- Spitz L, Kiely E, Sparnon T. Gastric transposition for esophageal replacement in children. Ann Surg. 1987;206: 69-73.
- Spitz L. Gastric transposition for oesophageal replacement. Pediatr Surg In. 1996;11:218-20.
- Spitz L, Kiely E, Pierro A. Gastric transposition in children-a 21 year experience. J Pediatr Surg. 2004;39: 276-81.
- Spitz L. Gastric transposition in children. Semin Pediatr Surg. 2009;18:30-33.
- Hamza AF. Colonic interposition in cases of esophageal atresia. Semin Pediatr Surg. 2009;18:40-43.
- Bax NMA, Rövekamp MH, Gunne AJPT. Early one-stage orthotopic jejunal pedicle-graft interposition in long-gap esophageal atresia. Pediatr Surg Int. 1994;9:483-85.
- Bax NMA, Zee DCVD. Jejunal pedicle graft for reconstruction of the esophagus in children. J Pediatr Surg. 2007;42:363-9.
- Bax NMA. Jejunal for bridging long-gap esophageal atresia. Semiin Pediatr Surg. 2009;18: 34-9.
- Lima M, Destro F, Cantone N, et al. Long-term follow-up after esophageal replacement in children: 45-Year single-center experience. J Pediatr Surg. 2015;1457-61.
- Cerchia E, Molinaro F, Pavone M, et al. Esophageal atresia with distal tracheoesophageal fistula: surgery treatment and a long term follow up. J Siena Acd Sci. 2012;4:30-4.
- Ein SH, Spitz L, Coran AC. Rob & Smith’s Operative Surgery: Pediatric Surgery. 1995;143-51.
- Ergun O, Celik A, Mutaf O. Two-stage coloesophagoplasty in children with caustic burns of the esophagus: hemodynamic basis of delayed cervical anastomosis: theory and fact. J Pediatr Surg. 2004;4:545-48.
- Angotti R, Molinaro F, Noviello C, et al. Gastric transposition as a valid surgical option for esohageal replacement in pediatric patients: experience from three Italian medical centers. Gastroenterol Rep. 2016;1: 5.
- Cauchi JA, Buick RG, Gornall P, et al. Oesophageal substitution with free and pedicle jejunum: short and long-term outcomes. Pediatr Surg Int. 2007;1:11-9.
- Gallo G, Zwaveling S, Bax KN, et al. A two-center comparative study of gastric pull-up and jejunal interposition for long gap esophageal atresia. J Pediatr Surg. 50:535-39.
- Arul GS, Parikh D. Oesophageal replacement in children. Ann R Coll Surg Engl. 2008;90:7-12.
- Othersen BH, Smith CD, Tagge EP. Esophageal replacement with colon in L. Spitz, A.C. Coran (Edn) “Rob & Smith’s Operative Surgery: Pediatric Surgery” V edizione, Chapman & Hall Medical. 1995;136-42.