- Biomedical Research (2014) Volume 25, Issue 2
Allogeneic bilayered tissue-engineered skin promotes full-thickness wound healing in ovine model
Ruszymah Bt Hj Idrus1,2*, Mohd Adha Bin P Rameli1, Low Kiat Cheong3, Law Jia Xian1,2, Chua Kien Hui2, Mazlyzam Bin Abdul Latiff4, Aminuddin Bin Saim1,51Tissue Engineering Centre, Universiti Kebangsaan Malaysia Medical Centre, 56000 Kuala Lumpur, Malaysia
2Department of Physiology, Faculty of Medicine, Universiti Kebangsaan Malaysia, 50300 Kuala Lumpur, Malaysia
3Laboratory Animal Resource Unit, Faculty of Medicine, Universiti Kebangsaan Malaysia, 50300 Kuala Lumpur, Malaysia
4Schools of Diagnostic & Applied Health Sciences, Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
5Ear, Nose & Throat Consultant Clinic, Ampang Puteri Specialist Hospital, Selangor, Malaysia
- *Corresponding Author:
- Ruszymah Bt Hj Idrus
Tissue Engineering Centre
Universiti Kebangsaan Malaysia Medical Centre
Jalan Yaacob Latif, Bandar Tun Razak
56000 Kuala Lumpur, Malaysia
Accepted date: December 02 2013
Abstract
Skin injuries can be treated via grafting. However, since both autologous and allogeneic skin grafts have their own limitations, skin tissue engineering was developed as an alternative approach to promote healing. Autologous tissue-engineered skin requires a few weeks for cell culture and cannot be used for acute treatment. In this study, healing potential of allogeneic bilayered tissue-engineered skin (BTES) was evaluated in sheep. Isolated allogeneic skin cells from the sheep were cultured using a combined medium of DKSFM:F12:DMEM in the ratio 2:1:1 supplemented with 5% FBS. Differential trypsinization was later carried out to separate the fibroblasts and keratinocytes. The allogeneic BTES was fabricated using allogeneic fibrin as biomaterial by polymerizing the fibrin-keratinocyte layer with calcium chloride followed by fibrin-fibroblast layer. Half of the wounds were isolated with PVC rings to prevent cell migration. The wounds were treated with allogeneic BTES and silk, as control. Wounds were observed at days 7, 14 and 21 to determine the rate of reepithelialization. After 3 weeks, the sheep were euthanized. Histological evaluation with H & E, elastin van Gieson and Masson’s trichrome staining showed that allogeneic BTES treated wound healed faster compared to the control group. In conclusion, allogeneic BTES has the potential to be developed as off the shelf product for rapid treatment of full thickness wound.
Keywords
Bilayered tissue-engineered skin, allogeneic, keratinocytes, fibroblasts, animal model
Introduction
Skin, the largest organ of the human body, composes of different types of tissue to carry out specific functions. Skin serves as a barrier to the external environment and involves in early defense against infection and dehydration [1,2]. Apart from that, skin also play requisite role in sensory reception, temperature regulation, metabolism and synthesis of vitamin D. Normal skin architecture can be disrupted by traumatic injuries or pathological changes such as reduce blood circulation, or diseases such as diabetes mellitus [3]. Loss of skin integrity because of injuries or illnesses may cause substantial physiologic imbal ance and in the worst scenario, significant disability or even death.
Autologous skin graft is the gold standard treatment for skin injuries. Autologous skin graft is preferable because it greatly reduces the risk of mortality and infection, shorten hospital stay and is more economical [4]. Cultured epithelial autograft was introduced 3 decades ago and functions well with patients suffering from extensive burn. This is due to the fact that the transplanted cells contribute to the formation of new epidermis. Nonetheless, in certain cases of extensive injury, difficulties arise when the patient is short of autograft donor sites and several weeks of culture is needed for the fabrication of autologous construct [5]. The use of allograft is one of the possible solutions but allograft is usually used as temporary cover. Advantages of allograft implantation include providing a bilayer structure identical to the destroyed tissue, reducing fluid and cellular losses, giving noble element protection and favoring early rehabilitation. Although allograft is readily available, concerns regarding the graft rejection and pathogen transmission render it less popular [6,7].
Skin tissue engineering began in the year 1975 following a successful serial cultivation of human keratinocytes by Rheinwald and Green [8]. Tissue-engineered skin can be fabricated using autologous or allogeneic cells [9]. Most of the commercially available tissue-engineered skins consist of allogeneic cells that are readily available and have minimum risk of rejection. Autologous tissueengineered skin is more suitable for the treatment of chronic wound but the long cell culturing process limits its immediate availability.
In this study, allogeneic plasma-derived fibrin, prepared in-house, was used to fabricate the construct. Fibrin is widely use in medical fields as haemostatic glue, as drug delivery vehicle and in tissue engineering as the biomaterial for the fabrication of constructs [10,11]. Fibrin helps wound healing by initiating hemostasis, provides a provisional matrix for cell migration and promotes angiogenesis [12]. Fibrin gel allows initial cell immobilization, hence minimizing cell loss and distribute the cells homogenously on wounds [13]. Due to its biological nature, fibrin can integrate with the patient’s skin and has excellent take rate.
Allogeneic BTES was fabricated by solidifying the fibrinkeratinocyte layer with addition of calcium chloride followed by a fibrin-fibroblast layer on top of a layer of silk that protects the wound and eases the handling. Through implantation, we investigated the feasibility of using allogeneic BTES for the promotion of full-thickness wound healing in ovine model.
Materials and Methods
Animals
Three adolescent male Marlin Siamese Long Tail sheep, aged 6 to 8 months, weighing about 10-15 kg at arrival were used. They were allowed to acclimatize for 2 weeks. All the sheep were housed individually and given ad libitum access to food and water. The animal protocol was approved by the Universiti Kebangsaan Malaysia Animal Ethical Committee (UKMAEC, certificate no. FISIO/ 2005/RUSZYMAH/12-JULY/146).
Skin cell isolation and culture
The animals were anaesthetized through intravenous injection with a mixture of Ketamax 100 (11 mg/kg, Marlab, Australia), ilium xylazil-100 (0.5 mg/kg, Troy Laboratories, Australia) and Zolatil 100 (0.05 mg/kg, Vicbac Laboratories, France) in the ratio 1:1:1 and atropine sulphate (0.2 mg/kg, Apes Laboratories, Australia). The biopsy sites on the rear leg of the sheep were shaved and washed with mild soap before sterilization with 70% alcohol and iodine poviderm. A full-thickness biopsy of size 1cm x 3cm was taken from each animal. The biopsy sites were sutured and covered with bactroban cream before bandaging with sterile gauze.
Harvested biopsies were stored at 4°C in Dulbecco’s phosphate buffered saline (DPBS) during transportation and digested with 0.3% collagenase type I for 6-8 h. This was followed by incubation in trypsin-EDTA (TE) for 5 min to obtain single cell suspension before trypsin inhibitor was added to neutralize the TE. The cells were cultured in 6-well plates using combined medium Define Keratinocytes Serum Free Medium:Ham’s F12: Dulbecco’s Modified Eagle Medium (DKSFM:F12:DMEM, 2:1:1) with 5% FBS (fetal bovine serum).
Fibroblasts were detached when the culture was 70% confluence by exposing the culture to TE for 5 min. Isolated fibroblasts were cultured using medium F12:DMEM supplemented with 10% FBS, while the keratinocytes were cultured with medium DKSFM. All the chemicals were purchased from Gibco (USA) except the ascorbic acid, which is from Sigma (USA).
Fabrication of bilayered tissue-engineered skin
Blood samples from the sheep were collected in vacutainers containing 3.2% sodium citrate (Bio-One, Grenier, USA). Collected blood samples were centrifuged for 5 min (650 g, 4°C) to isolate plasma. Isolated plasma was sterilized by passing through 0.2μm filter membrane (Sartorius, USA) to prevent spontaneous clotting. Isolated plasma was kept at -20°C.
The BTES was fabricated according to the protocol described by Mazlyzam et al. [14]. OtoSilk™ graft dressing (Boston Medical Products, US) was placed and affixed using ovine plasma at the bottom of 6-well plates. After that, keratinocytes were mixed with plasma and solidified with calcium chloride before the fibrin-fibroblast layer was fabricated on top.
Construct implantation
The implant areas in the anesthetized animals were shaved, cleaned and disinfected. A total of 8 fullthickness skin lesions were created on the dorsal side of the sheep. Four lesions were implanted with sterilized polyvinyl chloride (PVC) ring of diameter 3.6cm to prevent spontaneous wound healing. The lesions were covered with allogeneic BTES (4) and silk (4). Each type of construct covered 2 lesions with PVC rings and 2 lesions without PVC rings. For lesions without the PVC rings, the constructs were sutured with the native skin using Ethilon size 3/0, while for lesions with PVC rings, the ring was sutured using Ethilon 4/0 to the native skin before the constructs were inserted into the ring. The lesions were covered with gauze containing Bactroban and were affixed with Hypafix adhesive tape. The whole body of the animal was bandaged to protect the operation sites.
Postoperative management
Postoperative animals were housed individually with weekly dressing changes and wound site documentation. After 3 weeks, they were euthanized by intravenous administration of phenobarbital (60 mg/kg). The constructs and the surrounding tissue were harvested. The excised tissues were fixed in 10% formalin for 24 h and paraffinized for histological examination. The cross sections were prepared in thickness 5 μm from the central area of each specimen and stained with hematoxylin/eosin (H&E), elastin van Gieson and Masson’s trichrome.
Assessment of wound re-epithelialization
Re-epithelialization was quantified according to Geer et al. [15] using ImageJ software (version 1.47, National Institutes of Health). Re-epithelialization was defined as the cells-migration distance divided by the size of the wound. The wound size was measured as the distance from the wound edge to the point of incision, while the migration distance was measured on both sides of the wound by measuring the length of the basement membrane from the wound boundary to the most distant cells in the wound bed.
Statistical analysis
The data were analyzed using Statistical Package for Social Science (SPSS, version 20.0). Data were expressed as mean ± SEM. The differences were considered significant if p < 0.05.
Results
All sheep survived the experimental procedures without any complications. Implantation of PVC rings to the wounds did not cause any adverse reactions e.g. inflammation/ bleeding in the surrounding tissue. No signs of infection were seen during weekly dressing change.
Evaluation of re-epithelialization
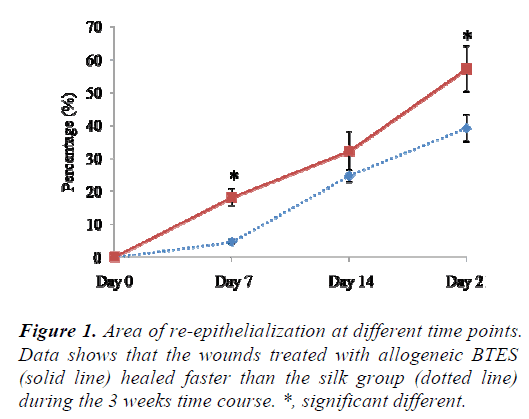
The wounds treated with allogeneic BTES re-epithelized faster than silk group at all time points. At day 7, 18.83 ± 2.11% of the allogeneic BTES group was re-epithelized compared to 4.62 ± 0.64% of the silk group (p < 0.05). At day 14, 32.16 ± 5.91% and 24.67 ± 1.95% of allogeneic BTES and silk treated wounds were covered with newly regenerated epidermis, respectively. By the time the sheep were euthanized at day 21, 57.20 ± 6.98% of allogeneic BTES group and 39.17 ± 4.05% of silk group were reepithelized (p < 0.05) (Fig. 1).
Gross examination
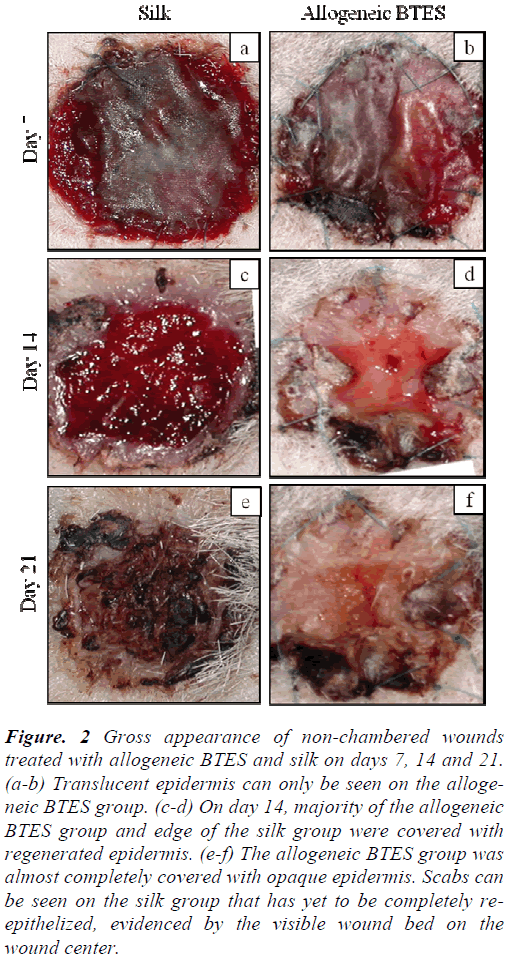
For both non-chambered and chambered wounds, the transplanted allogeneic BTES has excellent take rate. Non-chambered wounds treated with allogeneic BTES showed the presence of translucent epidermis by day 7. By day 14, most of the allogeneic BTES group was covered with opaque epidermis. No signs of epidermal regeneration were noted on the silk group on day 14. After 21 days of transplantation, the allogeneic BTES group was almost completely covered by regenerated epidermis while the silk group showed slow healing progression with the presence of less mature transparent epidermis and scab (Fig. 2).
Figure 2: Gross appearance of non-chambered wounds treated with allogeneic BTES and silk on days 7, 14 and 21. (a-b) Translucent epidermis can only be seen on the allogeneic BTES group. (c-d) On day 14, majority of the allogeneic BTES group and edge of the silk group were covered with regenerated epidermis. (e-f) The allogeneic BTES group was almost completely covered with opaque epidermis. Scabs can be seen on the silk group that has yet to be completely reepithelized, evidenced by the visible wound bed on the wound center.
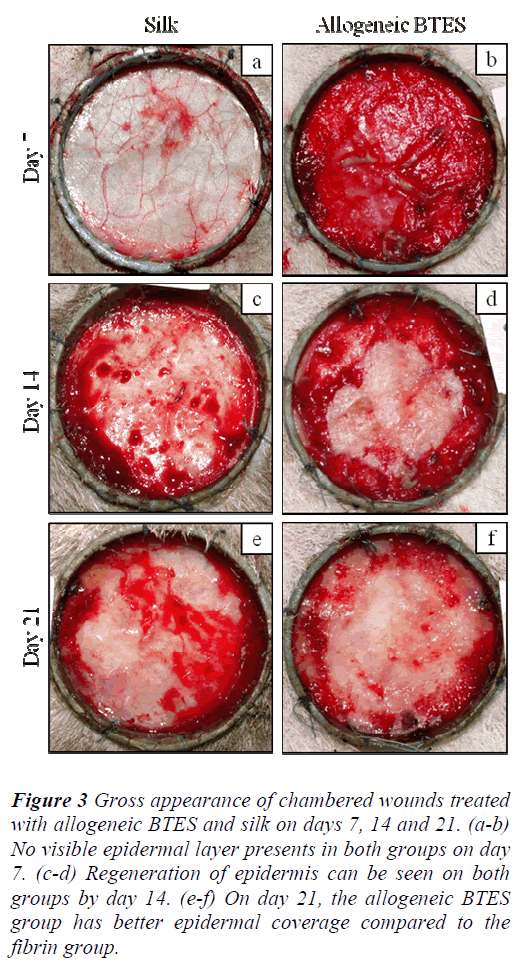
For the chambered wounds, none of the groups demonstrated the presence of epidermis by day 7. By day 14, regenerated epidermis became visible for all groups. The allogeneic BTES group has better epidermal coverage compared to the silk group on day 21 (Fig 3).
Figure 3: Gross appearance of chambered wounds treated with allogeneic BTES and silk on days 7, 14 and 21. (a-b) No visible epidermal layer presents in both groups on day 7. (c-d) Regeneration of epidermis can be seen on both groups by day 14. (e-f) On day 21, the allogeneic BTES group has better epidermal coverage compared to the fibrin group.
Hematoxylin and eosin staining
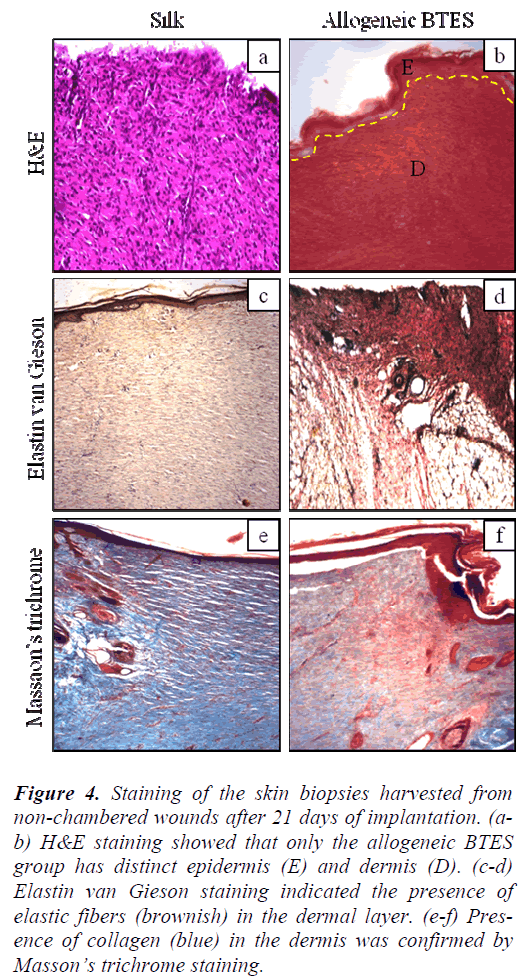
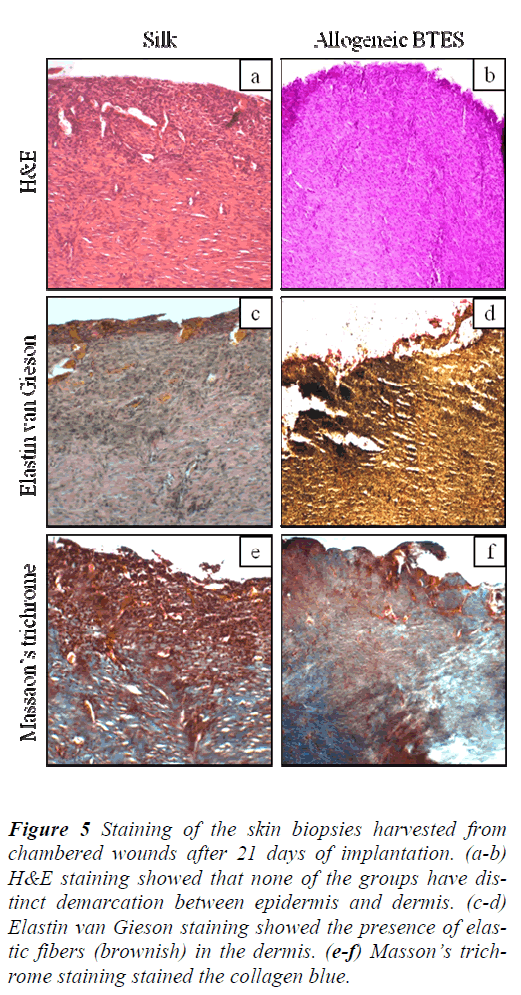
H&E staining showed that non-chambered wounds treated with allogeneic BTES have distinct demarcation between epidermis and dermis that was absence on the silk group (Fig. 4a-b). For the chambered wounds, none of the groups have distinct layers of epidermis and dermis (Fig. 5a-b).
Figure 4: Staining of the skin biopsies harvested from non-chambered wounds after 21 days of implantation. (ab) H&E staining showed that only the allogeneic BTES group has distinct epidermis (E) and dermis (D). (c-d) Elastin van Gieson staining indicated the presence of elastic fibers (brownish) in the dermal layer. (e-f) Presence of collagen (blue) in the dermis was confirmed by Masson’s trichrome staining.
Figure 5: Staining of the skin biopsies harvested from chambered wounds after 21 days of implantation. (a-b) H&E staining showed that none of the groups have distinct demarcation between epidermis and dermis. (c-d) Elastin van Gieson staining showed the presence of elastic fibers (brownish) in the dermis. (e-f) Masson’s trichrome staining stained the collagen blue.
Elastin van Gieson staining
The presence of elastic fibers, indicated by the brownish staining was spotted on all groups for both nonchambered and chambered wounds. The elastic fibers were found in the dermis (Fig. 4c-d & Fig. 5c-d).
Masson’s trichrome staining
Masson’s trichrome staining showed the presence of collagen on all groups for non-chambered and chambered wounds. The allogeneic BTES treated wounds have denser collagen compared to the silk groups (Fig. 4e-f & Fig. 5e-f).
Discussion
Tissue engineering is a promising approach employed for the development of skin substitutes to overcome the shortage of transplantable skin. A number of tissueengineered skin substitutes have been developed and one of it is MyDerm™ [16]. MyDerm™ is an autologous bilayered tissue-engineered skin substitute that composes of 2 layers, fibrin-keratinocyte and fibrin-fibroblast layers that mimic the native skin. MyDerm™ showed promising potential in the promotion of wound healing but it is more ideal for the treatment of chronic wounds as the culture of keratinocytes and fibroblasts require several weeks to obtain the required amount of cells. Another issue regarding MyDerm™ is the use of autologous fibrin as the biomaterial. Certain patients with extensive burn accompanied by significant fluid loss were unable to donate sufficient volume of blood for both cell culture and fabrication of fibrin construct.
The clinical use of allograft is restricted as they are inevitably immunologically rejected by non-immunosupressed patients 7-10 days post transplantation [17]. However, the skin substitutes comprised of cultured allogeneic keratinocytes and fibroblasts were well tolerated. The application of allogeneic tissue-engineered skin substitute is beneficial as the cells can promote wound healing, likely through the secretion of wound healing mediators before the allogeneic cells were replaced with native cells as the wound healed. Study has shown that allogeneic keratinocytes and fibroblasts could survive up to a month after implantation [18].
The rejection of allograft is due to the persistence of professional antigen presenting cells that include Langerhans cells, dermal dendritic cells and passenger leukocytes [19]. Tissue engineered skin substitutes consist of allogeneic keratinocytes and fibroblasts and are not rejected by the host because they are the “non-professional” antigenpresenting cells, by which, although they express the major histocompatibility complex (MHC) class I antigen on their cell surface, they do not constitutively express MHC class II antigen and common co-stimulatory molecules, such as B-7 and CD-40 [20,21]. Majority of the cells isolated from the skin are keratinocytes and fibroblasts, with a small proportion of melanocytes, Langerhans cells and passenger leukocytes. However, the isolated melanocytes, Langerhans cells and passenger leukocytes eventually diminish with serial cultivation. The loss of these cell types is crucial as they are the professional antigenpresenting cells that are capable of eliciting acute immune response.
In this study, wound healing potential of allogeneic keratinocytes, fibroblasts and fibrin fabricated construct was evaluated. Significant advantages of using allogeneic cells and fibrin fabricated construct include it is readily available and no collection of patient blood and skin biopsy were needed. Gross examination and histological staining clearly showed the allogeneic BTES group was superior compared to the silk group. Wounds treated with allogeneic BTES re-epithelized faster than the silk group at all time points without signs and symptoms of rejection. PVC ring was placed to isolate the wounds in order to prove the capability of allogeneic BTES to augment healing without the cell migration from the wound edge. Although the healing was not as good as the non-chambered wounds, however, allogeneic BTES was still capable of inducing the healing process. The slowdown in the healing of the chambered wounds is likely due to the inhibition of keratinocytes and fibroblasts migration from the surrounding native skin.
Allogeneic BTES was developed as permanent implant, unlike other allogeneic tissue engineered skin substitutes such as Biobrane™, Transcyte™, Apligraf™ and Dermagraft™ that need to be removed after a certain period of time [22]. The absence of signs and symptoms of rejection during the 3 weeks period supported the use of allogeneic BTES as permanent implant that will resolve automatically with time. At the same time, it also demonstrated that allogeneic cells and fibrin could be used to replace the autologous counterpart for the development of readily available skin substitute for the treatment of both acute and chronic skin injuries.
Conclusion
The allogeneic BTES has the potential to be developed as off the shelf skin substitute for rapid wound treatment. However, more studies should to be done to determine the minimum cultivation period or passage needed to eliminate the professional antigen presenting cells harvested together with the keratinocytes and fibroblasts to ensure the safety of the skin construct.
Acknowledgements
This work was financially supported by the research grant 06-02-02-0003-BTK/ER/022 and UKM-AP-2011-26.
References
- Ehrenreich M, Ruszczak Z. Update on tissueengineered biological dressings. Tissue Eng 2006; 12(9): 2407-2424.
- Ramos-e-Silva M, Boza JC, Cestari TF. Effects of age (neonates and elderly) on skin barrier function. Clin Dermatol 2012; 30(3): 274-276.
- Wohlrab J, Wohlrab D, Meiss F. Skin diseases in diabetes mellitus. JDDG 2007; 5(1): 37-53.
- Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface 2010; 7(43): 229-258.
- Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, et al. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003; 24(26): 4833-4841.
- Guerret S, Govignon E, Hartmann DJ, Ronfard V. Long-term remodeling of a bilayered living human skin equivalent (Apligraf®) grafted onto nude mice: immunolocalization of human cells and characterization of extracellular matrix. Wound Repair Regen 2003; 11(1): 35-45.
- Greaves NS, Iqbal SA, Baguneid M, Bayat A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen 2013.
- Rheinwatd JG, Green H. Serial cultivation of strains of human epidemal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975; 6(3): 331-343.
- Böttcher-Haberzeth S, Biedermann T, Reichmann E. Tissue engineering of skin. Burns 2010; 36(4): 450-460.
- Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface 2009; 6(30): 1-10.
- Praveen G, Sreerekha P, Menon D, Nair SV, Chennazhi KP. Fibrin nanoconstructs: a novel processing method and their use as controlled delivery agents. Nanotechnology 2012; 23(9): 095102.
- Clark RA. Fibrin and wound healing. Ann N Y Acad Sci 2001; 936(1): 355-367.
- Zheng Y, Ringe J, Liang Z, Loch A, Chen L, Sittinger M. Osteogenic potential of human periosteum-derived progenitor cells in PLGA scaffold using allogeneic serum. J Zhejiang Univ Sci B 2006; 7(10): 817-824.
- Mazlyzam A, Aminuddin B, Fuzina N, Norhayati M, Fauziah O, Isa M, et al. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns 2007; 33(3): 355-363.
- Geer DJ, Swartz DD, Andreadis ST. Fibrin promotes migration in a three-dimensional in vitro model of wound regeneration. Tissue Eng 2002; 8(5): 787-798.
- Mazlyzam A, Aminuddin B, Saim L, Ruszymah B. Living bilayered human skin equivalent: promising potentials for wound healing. Med J Malaysia 2008; 63(Suppl A): 32-33.
- Thivolet J, Faure M, Demidem A, Mauduit G. Cultured human epidermal allografts are not rejected for a long period. Arch Dermatol Res 1986; 278(3): 252-254.
- Phillips TJ, Manzoor J, Rojas A, Isaacs C, Carson P, Sabolinski M, et al. The longevity of a bilayered skin substitute after application to venous ulcers. Arch Dermatol 2002; 138(8): 1079.
- Benichou G, Yamada Y, Yun S-H, Lin C, Fray M, Tocco G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy 2011; 3(6): 757-770.
- Schurr MJ, Foster KN, Lokuta MA, Rasmussen CA, Thomas-Virnig CL, Faucher LD, et al. Clinical Evaluation of NIKS-Based Bioengineered Skin Substitute Tissue in Complex Skin Defects: Phase I/IIa Clinical Trial Results. Adv Wound Care (New Rochelle) 2012; 1(2): 95-103.
- Centanni JM, Straseski JA, Wicks A, Hank JA, Rasmussen CA, Lokuta MA, et al. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg 2011; 253(4): 672.
- Seet WT, Maarof M, Anuar KK, Chua KH, Irfan AWA, Ng MH, et al. Shelf-Life Evaluation of Bilayered Human Skin Equivalent, MyDerm™. PloS One 2012; 7(8): e40978.