- Biomedical Research (2016) Volume 27, Issue 3
Alkaloids from root tubers of Stephania kwangsiensis H.S.Lo and their effects on proliferation and apoptosis of lung NCI-H446 cells.
Ling Rong1,2, Dong Hu1*, Wan Wang1, Runpeng Zhao1, Xuewei Xu1, Wu Jing1*
1Department of Respiratory Medicine, The People’s Hospital of BoZhou.
2Medical Inspection Center, Anhui University of Science and Technology, Huainan, China.
- Corresponding Authors:
- Dong Hu
Department of Respiratory Medicine
The People’s Hospital of BoZhou No. 25
Middle Dongshan Road, Huainan Anhui 232001 P.R. China - Wu Jing
Department of Respiratory Medicine
The People’s Hospital of BoZhou No. 25
Middle Dongshan Road, Huainan Anhui 232001 P.R. China
Accepted Date: Dec 14, 2015
Abstract
To study the chemical constituents of Stephania kwangsiensis Lo. and to explore the effects of corydine on proliferation and apoptosis of lung adenocarcinoma NCI-H446 cells. Chemical structures were elucidated by MS, 1H-NMR and 13C-NMR. Effects of different concentrations of corydine on proliferation of lung adenocarcinoma NCI-H446 cells were determined by MTT assay. Effects of corydine on apoptosis rate of NCI-H446 cells were determined by flow cytometry. Three types of alkaloids were isolated from root tubers of Menispermaceae Stephania plant Stephania kwangsiensis Lo. Three different concentrations of corydine (20, 10, 5 Lg/ml) could all significantly increase the apoptosis rate of NCI-H446 cells after 48 h of treatment compared to the control group. Corydine can inhibit the proliferation of lung cancer NCI-H446 cells and induce their apoptosis.
Keywords
Stephania kwangsiensis Lo., corydine, small cell lung cancer, apoptosis
Introduction
Stephania kwangsiensis Lo. is a perennial deciduous herbaceous vine in the genus Stephania of the family Menispermaceae, which is mainly distributed in China's Guangdong, growing in mountain shrubs in limestone areas [1]. As a common Chinese herbal medicine, the root tuber extract of Stephania kwangsiensis Lo. is the main raw material of rotundine, which has analgesic, sedative, antipyretic effects and is thus widely used in clinical practice [2]. Stephania root tubers contain a variety of constituents such as alkaloids and terpenes [3,4]. Among them, alkaloids are the highest containing active constituent, with content reaching 3%~4% [5]. To further clarify its constituents, this study isolates three compounds from Stephania kwangsiensis Lo., namely: palmatine (1), corydine (2) and sinoacutine (3).
Small cell lung cancer is a highly malignant tumor, which is characterized by rapid growth, propensity to early systemic metastasis and higher incidence. Epidemiological [6,7] surveys have shown that small cell lung cancer accounts for 12%~15% of all lung cancers. Currently, small cell lung cancer is treated mostly by combined chemotherapy and radiotherapy in clinical practice. However, such therapy has large toxic side effects, where patients can hardly adhere to the treatment or have ineffective treatment. Therefore, the search for drugs with good efficacy and low toxic side effects is one major direction of cancer therapeutic research at present. According to studies in recent years, alkaloids are the main active constituents of Stephania kwangsiensis Lo. are alkaloids, which have antibacterial and insecticidal activities [8]. However, their effects on tumor cells have rarely been reported. This project studies the anti-human small cell lung cancer NCI-H446 cell activity of corydine, a main constituent in root tubers of Stephania kwangsiensis Lo., and explores its effects on proliferation and apoptosis of NCI-H446 cells, in order to provide experimental data and theoretical basis for clinical application of corydine in treatment of human small cell lung cancer.
Experimental Section
Chemistry
Reagents and instruments: API 4000 triple quadrupole LCMS system (Applied Biosystems, USA); AVANCE 600 superconducting actively shielded Fourier transform NMR spectrometer (TMS as internal standard, Bruker, Switzerland); N1100 rotary evaporator (EYELA, Japan); Sephadex LH-20 gel (GE, USA); ODS-A reversed phase silica gel (YMC, Japan); silica gel (Qingdao Haiyang Chemical Plant); chemical reagents (AR grade, Tianjin Weilong Chemical Reagents Co., Ltd.). Medicinal material was purchased from Fangzheng Pharmacy, which was identified by Associate Professor Wang Qingjia at School of Chinese Materia Medica, Chengdu University of TCM as the root tubers of Menispermaceae Stephania plant Stephania kwangsiensis Lo.
Extraction and isolation: Stephania kwangsiensis Lo. root tubers were ground with plant mill, and passed through a 60 mesh sieve. 10 kg dry powder was then weighed, placed in a glass container, added with 50 L of 80% ethanol, and extracted at room temperature for 48 h three times. After filtration, the three filtrates were combined, and concentrated under reduced pressure in a 50 water bath with rotary evaporator to yield an extract weighing 3.5 kg, which was then stored in a refrigerator for later use.
The extract was taken out, dissolved in water, and extracted sequentially with petroleum ether, ethyl acetate and n-butanol to give petroleum ether phase, ethyl acetate phase and nbutanol phase. The ethyl acetate phase was isolated and recrystallized by repeated normal phase silica gel, reversed phase silica gel and sephadex LH-20 column chromatographies to obtain three compounds.
Biology
Reagents and instruments: Corydine compound was prepared by the laboratory, while human lung cancer NCI-H446 cell lines (batch No.: 20150113) were provided by Shanghai Institute of Cell Biology, CAS. RPMI 1640 medium (GIBCO, USA, each L containing 0.33 mg of L-glutamine, 10% FBS, 100 U of streptomycin, 100 U of penicillin, pH 7.4); trypsin (MERCK, USA); AR grade H2O2 (Tianjin Weilong Chemical Reagents Co., Ltd., batch No.: 20150316); MTT (Sigma, USA); DMSO and FBS (provided by Nanjing Senbeijia Biotechnology Co., Ltd.); Annexin-V-FITC (PI) double staining reagent (provided by Nadika Biotechnology Co., Ltd.). Corydine was dissolved in DMSO, and then filtered and sterilized through a 0.22 μm nylon membrane to give a 100 mg/ml stock solution, which was stored in a -20°C refrigerator. The stock solution was diluted to the desired concentrations with RPMI 1640 medium before use, where the concentration of DMSO ≤ 0.1%. Flow cytometer (Thermo Fisher Scientific, USA).
Cell cultivation: Human small cell lung cancer NCI-H446 cells were cultured in a pH 7.4, 10% FBS containing RPMI 1640 medium under 37, 5% CO2 incubator conditions. Culture medium was replaced once every 48 h and the cells were passaged at a 1:5 ratio.
NCI-H446 cell proliferation inhibition rate: Logarithmic phase NCI-H446 cells were prepared into a 1 × 105/ml cell suspension with RPMI 1640 medium, seeded in 96-well plates at 200 μl per well, and cultured for 24 h. After cells were adherent, culture medium was aspirated off. 1) Treatment groups were added with different concentrations of corydine (final mass concentrations of 20, 10, 5 μg/ml), respectively, at 200 μl per well. 2) Control group was added with an equivalent volume of blank RPMI 1640 medium. Six replicate wells were set up for each group. After culturing for 24, 48, 72, 96 h as per the above routine cultivation method, respectively, MTT assay was performed. During the assay, each well was first added with 20 μl of MTT (5 mg/ml) and cultured for an additional 4 h, then supernatant was aspirated, each well was added with 150 μl of DMSO, and shaken for 10 min, followed by measurement of absorbance (A) value at 492 nm with a microplate reader. 3) Cell proliferation inhibition rate was determined by MTT assay; cell proliferation inhibition rate = (A value of control group - A value of treatment group) / A value of control group × 100%.
Determination of apoptosis rate: Logarithmic phase NCIH446 cells were prepared into a 1 × 106/ml cell suspension with RPMI 1640 medium, seeded in 24-well plates at 1 ml per well, and cultured for 24 h, then original medium was aspirated off. 1) Treatment groups were added with different concentrations of corydine (final concentrations of 20, 10, 5 μg/ml), respectively, at 1 ml per well. 2) Control group was added with an equivalent volume of blank RPMI 1640 medium. Three replicate wells were set up for each group. After culturing for 48 h, cells were collected by centrifugation, washed twice with pre-cooled PBS, and density-adjusted to 1 × 106/ml with binding buffer. Afterwards, 5 ml of the densityadjusted cells were placed into culture tubes, added with 5 μl of Annexin-V-FITC and 5 μl of PI, and reacted under dark conditions in the refrigerator at 4 for 30 min, followed by determination of apoptosis rate with flow cytometer.
Statistical processing: All the experimental data were expressed as mean ± standard deviation (X ± s), with 95% reference range. F-test, as well as chi-square test of apoptosis rate between different treatment groups were performed using SPSS 16.0 for Windows at a significance level α=0.05.
Results and Discussion
Chemistry
Structure elucidation
Compound 1: Bright yellow acicular crystals (methanol); soluble in water, methanol, ethanol, acetone, etc. 1H-NMR (600 MHz, DMSO-d6) δ: 7.05 (1H, s, H-1), 7.56 (1H, s, H-4), 3.29 (2H, t, J=6.4 Hz, H-5), 4.92 (2H, t, J=6.4 Hz, H-6), 9.74 (1H, s, H-8), 8.10 (1H, d, J=8.8, H-11), 8.02 (1H, d, J=8.8, H-12), 8.77 (1H, s, H-13), 4.18 (3H, s, COCH3), 4.12 (3H, s, COCH3), 3.88 (3H, s, COCH3), 3.94 (3H, s, COCH3); 13CNMR( 150 MHz, DMSO-d6) δ: 110.2 (C-1), 151.1 (C-2), 153.7 (C-3), 112.4 (C-4), 27.9 (C-5), 56.7 (C-6), 146.6 (C-8), 152.7 (C-9), 145.3 (C-10), 121.9 (C-11), 124.2 (C-12), 128.2 (C-13), 139.7 (C-14), 123.5 (C-15), 130.2 (C-16), 121.5 (C-17), 134.3 (C-18), 56.6 (C2-OCH3), 57.2 (C3-OCH3), 62.7 (C9-OCH3), 56.2 (C10-OCH3). The above data were basically consistent with the literature [9], so the compound was identified as palmatine.
Compound 2: Silver white acicular crystals (acetone); soluble in acetone, ethyl ether, ethyl acetate and chloroform. EIMS( m/z):341(M+), 326, 310, 155. 1H-NMR (600 MHz, DMSO-d6) δ: 6.71 (1H, s, H-3), 7.02 (1H, d, J=8.4, H-8), 7.15 (1H, d, J=8.4, H-9), 2.54 (3H, s, H-17), 3.71 (3H, s, COCH3), 3.83 (1H, s, COCH3), 3.92 (1H, s, COCH3); 13C-NMR(150 MHz, DMSO-d6) δ: 144.2 (C-1), 150.6 (C-2), 113.2 (C-3), 29.6 (C-4), 62.5 (C-5), 63.7 (C-6), 35.9 (C-7), 125.2 (C-8), 112.4 (C-9), 154.1 (C-10), 145.2 (C-11), 126.7 (C-12), 119.2 (C-13), 121.5 (C-14), 132.1 (C-15), 129.5 (C-16), 44.4 (C-17), 53.5 (C2-OCH3), 56.4 (C10-OCH3), 56.6 (C11-OCH3). The above data were basically consistent with the literature [10], so the compound was identified as corydine.
Compound 3: White cubic crystals (ethanol); soluble in methanol and ethanol. EI-MS(m/z): 327(M+), 312, 299, 284. 1H-NMR (600 MHz, DMSO-d6) δ: 6.82 (1H, d, J=8.4 Hz, H-1), 6.67 (1H, d, J=8.4 Hz, H-2), 6.30 (1H, s, H-5), 7.87 (1H, s, H-8), 3.72 (3H, s, COCH3), 3.90 (3H, s, COCH3), 2.43 (3H, s, NCH3); 13C-NMR( 150 MHz, DMSO-d6) δ: 123.5 (C-1), 111.7 (C-2), 148.2 (C-3), 146.5 (C-4), 120.1 (C-5), 165.3 (C-6), 183.8 (C-7), 123.3 (C-8), 62.5 (C-9), 34.1 (C-10), 131.2 (C-11), 124.8 (C-12), 45.4 (C-13), 151.9 (C-14), 38.5 (C-15), 48.1 (C-16), 56.5 (C3-OCH3), 55.2 (C6-OCH3), 41.4 (NCH3). The above data were basically consistent with the literature [11], so the compound was identified as sinoacutine.
Biology
Effects of corydine on NCI-H446 cell proliferation are shown in Table 1.
| Group | Mass concentration (μg/ml) | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|---|
| Control group | - | 0 | 0 | 0 | 0 |
| Treatment group | 20 | 48.39 ± 2.23** | 59.01 ± 4.71** | 75.12 ± 5.91 | 86.81 ± 7.99** |
| Treatment group | 10 | 31.34 ± 7.27* | 44.56 ± 4.36* | 59.87 ± 0.31* | 74.51 ± 3.22* |
| Treatment group | 5 | 24.85 ± 1.39* | 31.23 ± 2.12* | 40.11 ± 3.99* | 60.12 ± 3.19* |
Table 1: Effects of corydine on NCI-H446 cell proliferation (rate of cell apoptosis, ± s). Note: Comparison with the control group, *P<0.05, **P<0.01.
Effects of different mass concentrations of corydine on apoptosis rate of NCI-H446 cells are shown in Table 2.
| Group | Mass concentration (μg/ml) | Apoptosis rate (%) | Standard error |
|---|---|---|---|
| Control group | 0 | 1.02 | 0.41 |
| Treatment group | 20 | 8.77* | 1.29 |
| Treatment group | 10 | 9.12* | 2.01 |
| Treatment group | 5 | 12.38* | 2.12 |
Table 2: Effects of different mass concentrations of corydine on apoptosis rate of NCI-H446 cells (n=6). Note: Comparison with the control group, *P<0.05.
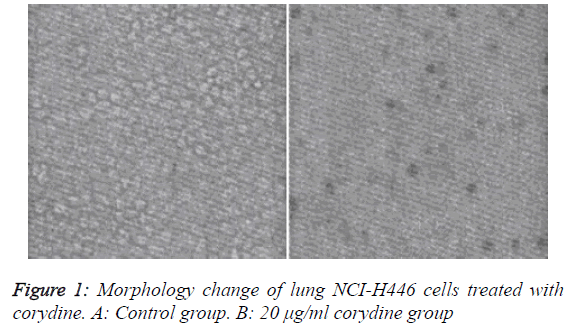
Changes in cell morphology are shown in Figure 1
We observed the morphology of lung adenocarcinoma N446 cells with an inverted optical microscope. Experimental results showed that corydine had some cytotoxic effect on A549 cells (Figure 1). In the non-treatment group, cells presented intact morphology, uniform size, clear outline and vigorous growth, all of which grew adherently. No contact inhibition was observed between cells, indicating that cells were in a very healthy state. After treatment with corydine solution, N446 cells exhibited obvious morphological changes, such as decrease in cellular volume and rounding and shrinkage of cells. Cells dramatically decreased in number, with almost no presence of viable cells. Light microscopic results demonstrated that corydine can effectively inhibit the growth of lung adenocarcinoma N446 cells and change their morphology markedly.
Stephania kwangsiensis Lo. is a Stephania plant in the family Menispermaceae, from whose root tubers bioactive substances having a variety of pharmacological actions [2] can be extracted, especially anti-tumor actions, since they have multitarget, multi-channel anti-tumor effects such as induction of tumor cell apoptosis, promotion of tumor cell differentiation, inhibition of intratumoral angiogenesis and suppression of tumor cell metastasis. Many studies have shown [12-14] that alkaloids like corydine, alone or in combination, can inhibit the proliferation of various tumor cells, and induce their apoptosis. These alkaloids have prominent cytostatic and proapoptotic effects on tumor cells. Moreover, their cytostatic and proapoptotic mechanisms are associated with the arrest of tumor cells in G1 phase, which causes cell cycle imbalance, thereby leading to stoppage of cell proliferation and occurrence of apoptosis.
Conclusion
The results of this study demonstrate that corydine has marked cytostatic and proapoptotic effects on human small cell lung cancer NCI-H446 cells, suggesting its potential value for clinical treatment of small cell lung cancer. This study only makes a preliminary exploration on the effects of corydine on proliferation and apoptosis of NCI-H446 cells, while the specific molecular mechanisms of its actions on human small cell lung cancer cells remain to be further studied.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- CAS Editorial Committee of the Flora of China, Flora of China (Volume XXX, Fascicule I). Beijing: Science Press 1996.
- Wang XK, Zhao TF. Distribution of alkaloids in Stephania plants and their biological activities. Chinese Pharmaceutical Journal1990; 25: 3-5.
- Min ZD,Zhong SM. Studies on the alkaloids of Stephaniakwangsiensis H.S. Lo (author's transl).ActaPharmaceuticaSinica 1980; 15: 532-537.
- HuangJM, Guo JX, Qu LB, Xiang BR. Note: Chemical Pattern Recognition of Three Chinese Herbal Medicines from the Genus StephaniaLour. Journal of Asian natural products research 1999; 1: 215-220.
- Min ZD,ZhongSM. Studies on the alkaloids of Stephaniakwangsiensis H.S. Lo. ActaPharmaceuticaSinica 1980; 15: 532-537.
- Bolster F, Crosbie I, O'Callaghan DS, Murray B, Kavanagh EC. Paraneoplastic limbic encephalitis and small-cell lung carcinoma. Journal of Thoracic Oncology 2015; 10: 852-853.
- Zehra Y, Vildan T, Guray C, Yusuf Y, Gulzade O, Ummugul U. Small Cell Lung Carcinoma with Overt Cutaneous Metastasis; Unusual Case. British Journal of Medicine and Medical Research2015; 6: 625-629.
- Atan MS, Dzulkefly KA, Aspollah SM, Anuar K, Vijay S. Isolation and Antibacterial Activity of Alkaloids from Phaeanthusopthalmicus. ASIAN JOURNAL OF CHEMISTRY 2011; 23: 3824-3826.
- Atan MS, Dzulkefly KA, Aspollah SM, Anuar K, Vijay S. Isolation and Antibacterial Activity of Alkaloids from Phaeanthusopthalmicus. ASIAN JOURNAL OF CHEMISTRY 2011; 23: 3824-3826.
- Soumitra H, Maidul H, Gopinatha SK. Studies on α-, β-, and γ-cyclodextrin inclusion complexes of isoquinoline alkaloids berberine, palmatine and coralyne. Journal of Inclusion Phenomena and Macrocyclic Chemistry 2014; 7: 311-323.
- Hu RL, Dai XJ, Lu YB, Pan YJ. Preparative separation of isoquinoline alkaloids from Stephaniayunnanensis by pH-zone-refining counter-current chromatography. Journal of Chromatography, B: Analytical Technologies in the Biomedical and Life Sciences 2010; 878: 1881-1884.
- Payo HA, Sandoval LD, Vélez CH, Oquendo SM,Alcaloides En La EspecieCubana Croton MicradenusUrb. RevistaCubana de Farmacia 2001; 35: 61-65.
- Wang F, Wang YH, Liu B, Liang YJ, Zhang BP. Effects of isocorydione on cell proliferation in Siha human cervical carcinoma cell lines. Chinese Journal of Clinical Oncology 2014; 8: 493-497.
- Qian P, Yang XW. Alkaloids from Zuojin Formula and their cytotoxicities against proliferation of cancer cells. Chinese Traditional and Herbal Drugs 2014; 1: 8-15.
- Wang R, Tang SA, Di HY,Duan HQ. Studies on anti-tumor metastatic constituents from Linderaglauca. China Journal of Chinese MateriaMedica 2011; 8: 1032-1036.