- Biomedical Research (2014) Volume 25, Issue 2
Alginate microencapsulation of stem cells as alternative source to the limited supply of donor tissue.
Tamader Y. Al-Rammah*Department of Radiological Sciences, College of Applied Medical Sciences, King Saud University, PO Box 66884, Riyadh 11586, Kingdom of Saudi Arabia and, College of Health and Rehabilitation Sciences, Princess Nora bint Abdulrahman PO Box 84428,University, Riyadh 11671, Saudi Arabia
- *Corresponding Author:
- Tamader Y. Al-Rammah
Department of Radiological Sciences
College of Applied Medical Sciences
King Saud University, PO Box 66884
Riyadh 11586, Kingdom of Saudi Arabia
E-mail: talrammah@ksu.edu.sa
Abstract
Microencapsulation is the procedure by which biologically active material is enclosed within micro spherical, semi permeable containers of a diameter between 0.2 to 3 mm. The microcapsules are usually produced by two-step procedure, in the first step the cells are immobilized within hydro gel micro beads and then the micro beads are covered with semi permeable membrane to obtain a microcapsule [1]. The encapsulation system is unique compare to other cell-based therapy techniques, because it enables the free exchange of nutrients and oxygen between the entrapped cells and their surrounding while preventing the escape and elimination of the entrapped cells. The microcapsuls can be injected at the transplantation bed, localizing the release of therapeutic factor. This approach avoids systemic side effects and increases patient’s compliance [2]. One of its advantages is the use of allogenic (nonhuman) cells as alternative source to the limited supply of donor tissue [3].
Keywords
Microencapculation; Mesenchymal stem cells; immunogenicity; Phenylananine hydroxylase (PAH)
Introduction
Microencapsulation of islets for transplantation was first introduced by Lim and Sun in 1980 using alginatepolylysine capsules. They have obtained prolonged survival of islets in vitro and in vivo by using a novel microencapsulation procedure which completely encloses viable islets within a semi permeable membrane. The microcapsular membrane composed of cross-linked alginate, a nontoxic polysaccharide, which is permeable to small molecules such as glucose or insulin but totally impermeable to large molecules such as immunoglobins or albumin [4].
Alginate-based cell encapsulation
Alginates are a family of unbranched anionic polysaccharides derived from brown algae (phaephyta) which occur extra-cellular and intra-cellular at approximately 20% to 40% of the dry weight. The 1,4-linked α-L-guluronate (G) and β-D-mannurante (M) are arranged in homopolymeric (GGG blocks and MMM blocks) or hetropolymeric block structure (MGM blocks)(5)The G/M ratio determines several main properties. Beads made from high G alginates are more stable and therefore more resistant to mechanical stress. Beads made of high M alginate bind more effectively with PLL, which has two advantages. First, the efficient binding at high M alginate with PLL can be used to decrease the capsule permeability, there by improving the immunoprotective properties of microcapsules.
Second, better PLL binding means less non-bound PLL on the outside of the capsules, there by reducing the risk of inducing fibrosis by positively charged PLL groups that are not well covered by the second alginate layer [5]. There are different materials applied for microcapsuls production such as polyethylene glycol [6], polyacrylates [7], agarose [8] and chitosan [9] but the most widely used microcapsules is the alginate-polylysine (PLL) capsule. This technique is based on the entrapment of individual cells in spherical alginate beads which is subsequently coated with a polycation. The PLL layer can be modified, which makes it possible to achieve many different grades of permeability [10,11].
Cell encapsulation technique
The encapsulation process starts with the formation of cell-containing alginate droplets which are produced by forcing alginate and cells to flow through a needle where the diameters of the droplets can also be controlled. Then the beads are formed by gellfication of the alginate in a calcium-rich medium. The beads are then provided with a poly-lysine membrane by simple suspending them in a poly-L-lysine (PLL) solution. During this step, PLL binds to mixed sequences of G and M in the alginate molecules. This induces the formation of complexes of the capsule surface consisting of alph-helical PLL surrounded by super helically orientated polysaccharide chains. The presence of these complexes decreases the porosity of the capsule. By varying the molecular weight and the concentration of the polylysine and the incubation time we can modulate the porosity of the capsule membrane. Then to provide biocompatibility, the capsules are suspended in a solution of alginate charged polylysine residues still present at the capsule surface [12].
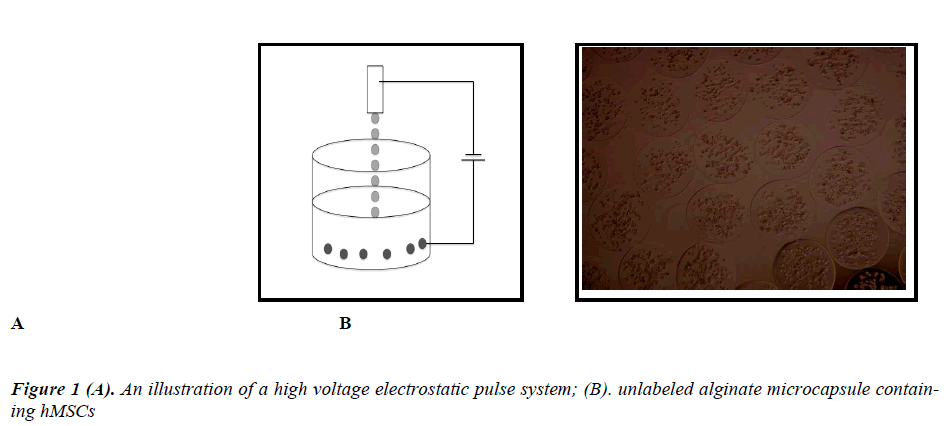
The two main techniques used to produce alginate droplets, either by air-driving technique which regulates air flow around the tip of the needle or by producing a highvoltage pulse around the tip of the needle which is known as the high voltage electrostatic pulse system [5,13-15].
Several techniques are applied to ensure smaller droplets production. The most popular are: coaxial air flow, where an air jet surrounding a nozzle increases the force available to break a hascent drop; vibrating jet breakage, where a liquid jet is being broken up into droplets due to a nozzle vibrations. Jet-cutter technology, where a jet is cut by an aeries of rotating knifes and the electrostatic droplet generation, where the reduction of the droplet size us caused by applying a high static potential between the nozzle and the jellifying bath.
The electrostatic droplet formation is one of the most precise methods, which enables one to manufacture spherical and uniform bead fractions with sizes from 3.0 mm down to 0.2 mm. The usage of the impulse voltage generator enables one to control and regulate process parameters like; the electric voltage V, the impulse frequency ƒ and the impulse duration time τ which plays a crucial role in the droplet formation. Low efficiency of the electrostatic process can be significantly improved by multiplication of the nozzles, without any loss of the micro beads quality. Where the efficiency of this device could reach 700 ml/h in comparison with about 60 ml/h for the single nozzle device [16].
Contrast enhanced capsules
Barnett et al have developed for the first time radio opaque capsules that can be used for encapsulated of cells and allow real time x-ray guided delivery as well as noninvasive x-ray follow-up imaging. The authors modified the classical alginate/poly-L-lysine/alginate (APA) microencapsulation protocol. They used electrostatic droplet generator to encapsulate human islet cells, they also added barium or bismuth to the inner core layer of high guluronate alginate (Protanal) that surrounds the islets. The outer layer of the APA capsule, made with high mannuranate alginate (Keltone), was added without contrast in order to avoid any potential inflammatory or toxic reaction induced by the radio opaque contrast agent. They have chosen high guluronate alginate for the inner alginate layer because of its superior strength. For the outer layer they chose a high mannuronate alginate as it has been shown to be less immunogenic.
In this approach the contrast agent is contained in the capsules and not the cells, therefore avoiding the dilution effect of cell division and may also bypass potential toxicity issues that result from direct cell labeling. They have demonstrated that these x-Caps can be readily visualized at a single capsule level.
Therefore, these capsules deliver cellular therapeutics with significant radio opacity to assess cellular position [17].
Application
The strategy of cells microencapsulation has been used preferentially with the following therapeutic purposes: 1) development of bioartificial organs, such as the pancreas, 2) treatment of classical mendelian disorders caused by an enzymatic or gene product deficiency, such as dwarfism, hemophilia and Tysosomal-storage disease, 3) Cancer eradication, 4) treatment of other disorders, such as degenerative diseases of the CNS and to reduce urea levels in renal failure [18].
Generally speaking there are two basic areas of medical applications of the microencapsulated cells: the first one, as very sophisticated drug delivery systems with continuous and controlled release of therapeutic agents. In this case the microcapsules could be implanted into body directly of the target site and could be especially effective for treatment of cancer and neurological diseases (e.g. Alzheimer’s, Parkinson’s and Huntington’s disease). Several approaches have been used to apply the encapsulation technology in cancer therapy, such as enhancing the immunogenicity of tumor cells and angiogenesis inhibition. Another approach used encapsulated cells overexpressing enzymes that can activate chemotherapeutic agents to deliver vaccines as another concept in canver therapy where delivery of antibodies by encapsulated cells could be an alternative and more cost-effective method. Studies suggest that microencapsulated stem cells may have a much greater potential for heat regeneration in comparison to free stem cells. Reports also indicated that supplementation of encapsulated angiogenic factors can stimulate new blood vessel growth (neuvascularization) and restore perfusion in damaged or ischemic myocardium [18].
In addition, encapsulated cells can also be genetically modified to express any desired protein in vivo without changes in the patient’s genome. This therapeutic mode might allow the quality assessment before implantation and eliminates the tedious preparation and formation process that is usually associated with the more traditional peptide delivery systems. Cell microencapsulation also has implications for gene therapy, in that cells can be genetically engineered to secrete a desired genetic product prior to this encapsulation. They are also being explored as a therapeutic tool for an in vivo gene therapy approach [19].
The second field of medical application of the microencapsulated cells is a replacement or support of organ functions. In this case the microencapsuls could be implanted into patients body, for example as a hybrid pancreas or could work as a part of extracorporeal devices, for instance as bioreactor with hepatic cells for liver support. The microcapsule, which contains living cells, is also well-known as, so called “an artificial cell”. Where living cells are immobilized inside a spherical matrix, and surrounded by semi permeable membrane. The main task of the matrix is a creation of the best possible living conditions for encapsulated cells. The main task of the membrane is immunoisolation of the encapsulated cells from the destruction by the immunological system of the host. So the membrane has to protect the capsule interior from the penetration of antibodies and leukocytes. The microcapsules have to ensure free oxygen and nutrients transport from the environment to the capsule interior and a reverse transport of metabolites (waste products) and therapeutic substances, produced by the encapsulated cells. The matrix and the membrane have to be highly biocompatible [16].
Several approaches have been used to apply the encapsulation technology in cancer therapy, such as enhancing the immunogenicity of tumor cells and angiogenesis inhibition. Another approach used encapsulated cells overexpressing enzymes that can activate chemotherapeutic agents to deliver vaccines as another concept in canver therapy where delivery of antibodies by encapsulated cells could be an alternative and more cost-effective method. Microencapsulation has been used as an alternative to directly injecting hepatocytes, creating a living cellbased replacement system [19].
It was reported that cell encapsulation technique was used to engineer cells to provide human cilliary neurotrophic factor (CNTF) a factor that is protective of photoreceptor cells. They encapsulated the cells in a proprietary capsule. They implanted these capsules into one eye of dogs with the rcd1 model of canine retinitis pigmentosa. The capsules were in place for seven weeks, early in the life cycle of the dog, in which time most 50%of the photoreceptor cell degeneration take place. After seven weeks, the eyes with the implants showed an increased survival at photoreceptor cells, compared with the untreated cells [20].
Encapsulation of Phenylananine hydroxylase (PAH) expressing cells is a potential new therapy for maternal Phenylketonuria (PKU) a disease causes congenital malformation and mental retardation in children [21].
Studies confirmed in vitro hMSC were able to differentiate along the chondrogenic lineage when encapsulated in Ca-alginate microcapsules and stimulated with TGF-β3. Other studies done by Peak et al used the approach based on genetically engineered cells to release growth factors for cartilage regeneration [18].
Successful studies employing immobilized primary cells include the extracorporeal immunoisolation of hepatocytes and the manroencapsulation of adrenal cells for the treatment of chronic pain and allotransplantation of microencapsulated parathyroid tissue.
Challenges and causes of graft failure
One of the technique challenges involves the production of uniform capsules with excellent repeatability and reproducibility both within and between batches. The adoption of automated machines for microencapsulation could result in improved reproducibility in terms of shape, size and morphology.
The choice of transplantation site is another important consideration. It is necessary to weigh issues such as the safety and possibility of re-transplantation (peritoneal cavity, subcutaneous transplantation) against proximity to the circulation (intrahepatic transplantation or membranes supporting vascularization) [22].
In general causes of microencapsulation graft failure can be summarized in; 1) lack of biocompatibility, 2) incomplete immunoprotection, 3) limited supply of oxygen which leads to hypoxia.
Bioincompatibility of the capsules can be improved by; 1) increasing purity of the alginate, 2) reducing capsules diameter from 800 to 500 μm, 3) the composition of alginate. Alginates are composed of mannuronic acid (M) and guluronic acid (G). The biocompatibility was better when alginate was used with an intermediate than with a low or high G content. Hypoxia leads to dysfunction and loss of vitality, it is reported to induce both necrosis and apoptosis and the proportion of these two modes depend on the cell type, the duration and the severity of the hypoxic stress.
Human mesynchemal cell encapsulation
Mesenchymal stem cells are easily isolated from adult bone marrow, adipose tissue, cord blood, placenta and umbilical cords, fetal organs such as liver, blood, bone marrow and lungs. MSC can be expanded ex vivo with out differentiation or maturation and can be modified genetically to express a variety of genes.
MSC are hypo immunogenic, they are poorly recognized by human leukocyte antigen (HLA)-incompatible hosts. MSC suppress cytotoxic or helper cells.
hMSCs can maintain their stem-cell properties in the microcapsules, at 4 weeks post-encapsulation 80-85% of encapsulated hMSCs still express the common mesenchymal marker: CD 105, CD 90, CD 29, CD 44. Free MSCs can be engineered genetically for cells-based therapy of different illnesses including cancer.
Penolazzi et al reported their experience in successfully encapsulating Wharton’s Jelly Mesenchymal Stem Cells in alginate micro beads where they concluded that alginate does not prevent cell functionality, on the contrary in some cases it many promote it. WMJ MSCs have been shown to share properties of both bone marrow MSCs and embryonic stem cells [23].
Conclusion
In conclusion from the above studies, Microencapculation cells promises a new light for stem cells therapy and technique. It is easy to implemented and genetically modified to express any desired protein in vivo without changes in the patient’s genome. It is also can be expanded ex vivo without segregation or maturation. The benefits of Microencapculation prevent systemic side effects and decreases patients’ compliance. It is also decreases and limits the use of donor tissue.
References
- Zimmermann H, Shirley SG, Zimmermann U. Alginate-based encapsulation of cells: past, present, and future. Curr Diab Rep 2007; 7: 314-320.
- Goren, A., Dahan, N., Goren, E., Baruch, L. & Machluf, M. Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. Faseb J (2010) 24 (1): 22-31
- Freimark, D., et al. Use of Encapsulated Stem Cells to Overcome the Bottleneck of Cell Availability for Cell Therapy Approaches. Transfus Med Hemother 2010; 37 (2): 66-73.
- Lim Franklin & Anthony, S. Microencapsulated Islets as Bioartificial Endocrine Pancreas. Science 210, 908-910 (1980).
- de Groot, M., Schuurs, T.A. & van Schilfgaarde, R. Causes of limited survival of microencapsulated pancreatic islet grafts. J Surg Res 2004; 121: 141-150.
- Cruise, G.M., et al. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant 1999; 8: 293-306.
- Sefton, May, Lahooti & Babensee. Making microencapsulation work: Conformal coating, immobilization gels and in vivo performance. J Control Release 2000;65: 173.
- H. Iwata, et al. Evaluation of microencapsulated islets in agarose gel as bioartificial pancreas by studies of hormone secretion in culture and by xenotransplantation. Diabetes 1989; 38: 224.
- Zielinski, B.A. & Aebischer, P. Chitosan as a matrix for mammalian cell encapsulation. Biomaterials 1994;15, 1049-1056.
- Goosen MF, O'Shea GM, Gharapetian HM, Chou S, Sun AM. Optimization of microencapsulation parameters: Semipermeable microcapsules as a bioartificial pancreas. Biotechnol Bioeng 1985; 27: 146-150.
- Rokstad A., et al. Microencapsulation of cells producing theraputic proteins: Optimizing cell growth and secreation. Cell Transplant 2002; 11: 313.
- van Schilfgaarde R, & P., d.V. Factors influencing the proerties and performance of microcapsules for immunoprotection of pancreatic islets. J Mol Med 1999; 77: 199-205.
- Wolter GHJ, Fritschy WM, Gerrits D & van Schilfgraad R. A versatile Alginate droplet generator applicable for microencapsulation of pancreatic islets. J Appl Biomat 1992; 3: 281-286.
- Halle JP, et al. Studies on small (<300 microns) microcapsule: II Parameters governing the production of alginate beads by high voltage electostatic pulses. Cell Transplant 1994; 3: 365-372.
- Hsu BR, Chen HC, Fu SH & Huang YY. The use of feild effects to generate calcium alginate microspheres and its application in cell transplantation. J Formos Med Assoc 93 1994; 240-245.
- Lewinska Dorota, Bukowski Jozef, Kozuchowski Marek & Kinasiewicz Andrzej Kinasiewicz, W.A. Electrostatic Microencapsulation of Living Cells. Biocybernetics and Biomedical Engineering 2008; 28: 69-84.
- Barnett, B.P., et al. Radiopaque alginate microcapsules for X-ray visualization and immunoprotection of cellular therapeutics. Mol Pharm 2006; 3: 531-538.
- Orive Gorka, Gascon Alicia, Hernandez Rosa, Igartua Manuela & Jose, P. Cell microencapsulation technology for biomedical purposes: novel insights and challenges. TRENDS in Pharmacological Sciences 24, 207-210 (2003).
- Orive G, et al. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol 2004; 22: 87-92.
- Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther 2006; 6: 717-726.
- Santillan DA, Santillan MK, Hunter SK. Cell encapsulation as a potential nondietary therapy for maternal phenylketonuria. Am J Obstet Gynecol 2009; 201(3): 289 e1-6.
- Orive G, et al. Cell encapsulation: promise and progress. Nat Med 2003; 9: 104-107.
- Penolazzi L, et al. Encapsulation of mesenchymal stem cells from Wharton's jelly in alginate microbeads. Tissue Eng Part C Methods February 2010, 16: 141-155.