Research Article - Biomedical Research (2017) Volume 28, Issue 8
Alcoholic extract of Tarantula cubensis induces apoptosis in MCF-7 cell line
Ayse ER1, Orhan Corum2, Duygu Corum3, Mustafa Hitit4*, Huseyin Donmez5 and Aydn Guzeloglu4
1Department of Pharmacology and Toxicology, Veterinary Faculty, Selcuk University, Konya, Turkey
2Department of Pharmacology and Toxicology, Veterinary Faculty, Dicle University, Diyarbak?r, Turkey
3Arac Rafet Vergili Vocational School, Kastamonu University, Kastamonu, Turkey
4Department of Animal Genetics, Veterinary Faculty, Selcuk University, Konya, Turkey
5Deparment of Medical Genetics, Medicine Faculty, Selcuk University, Konya, Turkey
- *Corresponding Author:
- Mustafa Hitit
Department of Animal Genetics
Veterinary Faculty
University of Selcuk, Turkey
Accepted on January 10, 2017
Abstract
Tarantula cubensis Alcoholic Extract (TCAE) is a homeopathic agent used for treating many disorders. This study aimed to define the effects of TCAE on the breast carcinoma cell line (MCF-7). After various concentrations (10, 20, 40, 80 and 160 μl/ml) of TCAE were applied to MCF-7 cells and the human embryonic kidney cell line (HEK293), the cells were incubated for 1, 3, 6, 9, 12, 24 and 48 h, followed by analysis by MTT assays. According to the results of the MTT assays, cells treated with 20 or 40 μl/ml TCAE for 6 h were applied to apoptosis analysis by flow cytometry. Secreted levels of tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, IL-6, IL-10, Interferon-γ (IFNγ), Transforming Growth Factor- β (TGFβ), and Nuclear Factor-kappa B (NF-κB) were measured using ELISAs. TNFα and TGFβ levels increased while IL-6 and IL-10 levels fluctuated in MCF-7 cells. In conclusion, our study suggests that TCAE may change the normal cancer physiology and lead to cell death by activating apoptosis in MCF-7 cells.
Keywords
Tarantula cubensis alcoholic extract, Theranekron, Cytokines, MCF-7, Apoptosis.
Introduction
Tarantula cubensis Alcoholic Extract (TCAE) is a homeopathic remedy prepared from the spider Tarantula cubensis. The whole spider is processed and diluted with 60% alcohol for potentiation according to the rules of the “Pharmacopeia Germanica” [1]. In veterinary medicine, it is often used as an epithelialization accelerator and demarcation provider because of its oedema-relieving effects in trauma, necrotic disorders, and many infectious diseases [1-3]. TCAE also has an anti-inflammatory effect [1]. Administration of TCAE leads to regression of mammary tumors, and postoperative use of TCAE prevents the reoccurrence of tumors in dogs [4]. In addition, TCAE is effective for the treatment of oral ulcers and papillomas [5,6].
Breast cancer is frequently observed among women and is the fourth most common cause of cancer deaths [7]. One of the etiologic factors of breast cancers is chronic inflammation [8]. Cytokines play crucial roles in inflammation and are produced by tumor cells [9], which are associated with malignancy, the tumor stage, and survival [10]. They are the most significant players in tumor initiation, promotion, angiogenesis, and metastasis [10].
Nuclear factor-kappa B (NF-κB) is a major survival factor that regulates cellular processes such as cell adhesion, immune responses, apoptosis, and proliferation. It has a regulatory role in the balance of apoptosis-proliferation in tumor cells. Moreover, the expression of inflammatory cytokines is induced by NF-κB, and in turn, these cytokines induce activation of NF-κB. Inhibition of NF-κB can improve the efficacy of cancer therapies [11,12]. Studies have shown that NF-κB plays a role in the transition from chronic inflammation to cancer [13].
Tumor Necrosis Factor-α (TNFα) is a multifunctional proinflammatory cytokine that plays important roles in the proliferation, differentiation, survival, and death of cells [14]. It is an angiogenic stimulator that promotes tumor cell migration and invasion [15,16]. Local administration of a high dose of TNFα selectively devastates blood vessels in tumors, thus showing potent anticancer effects. However, upon chronic production, TNFα acts as an endogenous tumor-promoting agent that enhances tumor growth and spread [17]. Interleukin (IL)-1 promotes tumor proliferation, invasiveness, angiogenesis, and metastasis. Because IL-1 receptor antagonist (IL-1Ra) inhibits IL-1 functions, it decreases tumor invasiveness and can be used in cancer therapy [18,19]. IL-6 is a prognostic factor in breast carcinomas and plays an important role in the development, invasion, proliferation, apoptosis, and angiogenesis of tumors. Moreover, IL-6 is a significant contributor to tumor cell survival and drug resistance. Conversely, expression of IL-6 is not induced by breast cancer cells sensitive to drugs treatments, while its expression is produced by breast cancer cells resistant to drugs treatments [20]. IL-10 has an antitumor effect in cancer and displays a tumor regression activity [21].
Transforming Growth Factor-β (TGFβ) is a major regulator in many cellular processes such as proliferation, differentiation, migration, and apoptosis. TGFβ has dual functions in tumor progression [22]. TGFβ as a tumor suppressor has antiproliferative effects in the early stages of tumorigenesis, but tumor cells become resistant to this effect in later stages [22,23]. Blocking TGFβ improves the intratumoral penetration of chemotherapeutic drugs and decreases angiogenic gene expression [24].
Interferon-γ (IFNγ) is a potent immunomodulatory, antiviral, and anti-proliferative cytokine. In addition, it inhibits tumor angiogenesis and shows an anticancer activity. IFNγ increases cellular susceptibility to apoptosis in tumor cells [25].
The homeostasis of cell proliferation and death is regulated by apoptosis in multicellular organisms. Therefore, treatments that affect the apoptotic threshold can change the natural progression of certain diseases such as cancer [26]. Considering these facts and the effects of TCAE on cancer, papilloma, and ulcers in clinical trials [4-6], it has been hypothesized that cancer physiology may be affected by TCAE.
The aim of the present study was to determine the effects of TCAE on cell proliferation, and apoptosis, NF-κB expression, and secreted cytokine levels in the MCF-7 cell line, because NF-κB, TNFα, IL-1β, IL-6, IL-10, TGFβ, and IFNγ have important roles in cancer.
Materials and Methods
Agents and cell culture
TCAE (Theranekron D6® Enj. Sol. Richter Pharma, Austria), breast cancer cell line (MCF-7) and the human embryonic kidney 293 (HEK293) cell line were used in this study. Cell lines were obtained from the American Type Culture Collection (ATCC).
Thiazolyl blue tetrazolium bromide (MTT) assay
To determine the cytotoxic effect of TCAE, we performed MTT assays. For the MTT assay, 5 × 103 cells in 100 μL culture medium were added to each well of a 96-well microplate and incubated for 24 h at 37°C with 5% CO2. The medium was removed and the cells were washed twice with PBS. To equalize the mitosis cycle, the cells were incubated in serum-free medium for 24 h (starvation). Then, the cells were treated with 100 μL culture medium containing various concentrations (0, 10, 20, 40, 80, and 160 μl/ml] of TCAE for 1, 3, 6, 9, 12, 24 and 48 h. At the end of each time point, 10 μL of a 12 mM MTT solution (#M2128; Sigma-Aldrich) was added to each well, followed by incubation for 4 h at 37°C with 5% CO2. After the medium was removed, 50 μL DMSO was added to each well, followed by incubation for 10 min at 37°C with 5% CO2. The blue crystals were dissolved by gentle pipetting after incubation, and then absorbance values were measured on an ELISA reader (MWGt Lambda Scan 200, Bio- Tek Instruments, Winooski, VT, USA) at 490 nm.
Apoptosis analysis
Because TCAE-induced cytotoxicity induces different cell death pathways, such as apoptosis and necrosis, we applied Annexin V staining and flow cytometry. Annexin V distinguishes apoptotic cell death. About 1 × 106 cells were seeded into a flask with 3 ml medium containing 10% fetal bovine serum and 1% penicillin/streptomycin. After the cells were treated with optimal TCAE concentrations (20 and 40 μl/ml) and times (6 h) determined by the MTT assay, 5 μL Annevin V-FITC (eBioscience ABD #BMS500FI/20) was added to each well. Cells were collected by trypsinization. The cells were incubated for 10 min at room temperature, washed with Binding Buffer (1X), and then stained with 10 ml propidium iodide (eBioscience ABD, #BMS500FI/20) (20 μg/ ml). Analysis was performed with a cell sorter (FACSAria III, BD Biosciences).
Cytokine analysis
Supernatants were obtained from MCF-7 cell treated with or without TCAE for 2, 4, 6, 8, 10 and 24 h. Secreted levels of NF-κB, TNFα, IL-1β, IL-6, IL-10, TGFβ, and INFγ were determined by ELISAs (eBioscience, San Diego, CA, USA) as described by the manufacturer’s instructions. Plates were read using the ELISA reader.
Statistical analysis
Intra-group MTT assay and apoptosis analyses were evaluated by dependent t-tests (MINITAB® Release 14). P<0.05 was considered as statistically significant. Cytokine levels were evaluated by analysis of variance and Duncan’s post-hoc test (SPSS 19.0). P<0.05 was considered as statistically significant.
Results
MTT assay
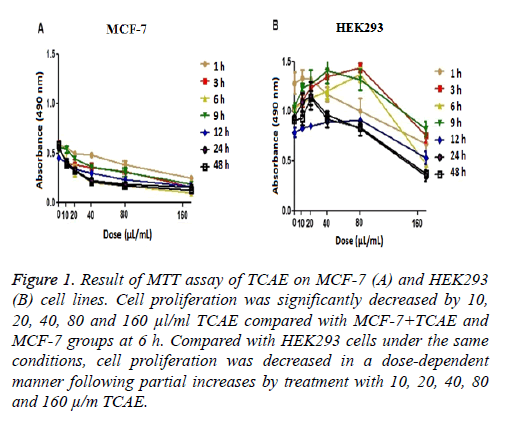
To determine the optimal treatment time and TCAE concentration for further analyses, MTT assays of MCF-7 and HEK293 cell lines were performed using various TCAE concentrations (0, 10, 20, 40, 80, and 160 μl/ml) for different times (1, 3, 6, 9, 12, 24, and 48 h) (Figure 1). TCAE showed an inhibitory effect on the proliferation of MCF-7 cells in concentration- and time-dependent manners.
Cell proliferation was significantly decreased by 10, 20, 40, 80 and 160 μl/ml TCAE compared with MCF-7+TCAE and MCF-7 groups at 6 h (Figure 1). Compared with HEK293 cells under the same conditions, cell proliferation was decreased in a dose-dependent manner following partial increases by treatment with 10, 20, 40, 80 and 160 μl/ml TCAE (Figure 1). MTT assay was repeated at least 5 times in groups.
Figure 1: Result of MTT assay of TCAE on MCF-7 (A) and HEK293 (B) cell lines. Cell proliferation was significantly decreased by 10, 20, 40, 80 and 160 μl/ml TCAE compared with MCF-7+TCAE and MCF-7 groups at 6 h. Compared with HEK293 cells under the same conditions, cell proliferation was decreased in a dose-dependent manner following partial increases by treatment with 10, 20, 40, 80 and 160 μ/m TCAE.
Apoptosis analysis
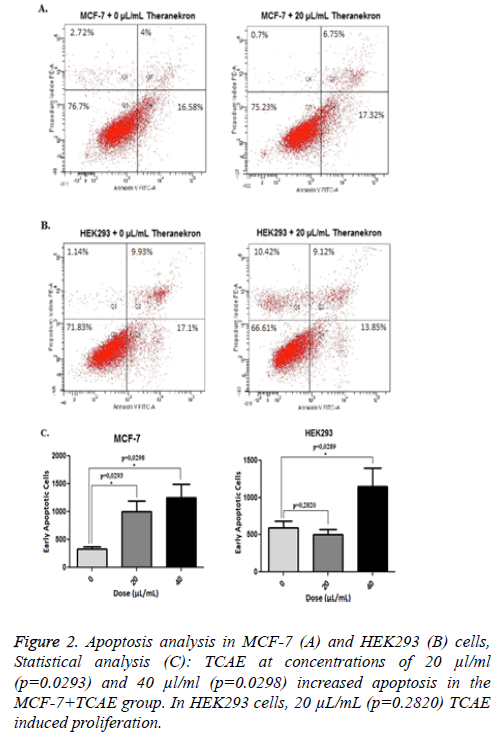
According to the results obtained from the MTT assay, treatment with 20 and 40 μl/ml TCAE for 6 h was applied for the apoptosis analysis. TCAE at concentrations of 20 μl/ml (p=0.0293) and 40 μl/ml (p=0.0298) increased apoptosis in the MCF-7+TCAE group. In HEK293 cells, 20 μl/ml (p=0.2820) TCAE induced proliferation, whereas 40 μl/ml (p=0.0289) TCAE increased apoptosis (Figure 2). TCAE at 20 μl/ml induced the lowest apoptosis rate in HEK293, whereas same dose of TCAE induced distinct apoptosis in MCF-7 cells; hence TCAE at 20 μl/ml was used to evaluate secreted cytokine levels.
Cytokine analysis
Effects of 20 μl/ml TCAE on NF-κB, TNFα, IL-1β, IL-6, IL-10, TGFβ and IFNγ levels are shown in Table 1. Fluctuations in IL-6 and IL-10 levels, and increases in TNFα (24 h) and TGFβ (24 h) levels were found in the MCF-7 group (P<0.05). TCAE administration did not change the secreted cytokine levels of MCF-7 cells (P>0.05). To evaluate the general effect of TCAE on all cytokines, the Area under the Curve (AUC) was calculated. Therefore, we compared the AUC values of MCF-7 and MCF-7+TCAE groups. TCAE decreased the AUC values of NF-κB (-7.02%), TNFα (-87.33%), IL-6 (-34.40%), and TGFβ (-5.47%), and increased the AUC values of IL-1β (5.22%), IL-10 (23.85%), and IFNγ (70.38%).
Discussion
In veterinary medicine, TCAE is a homeopathic agent that is often used as an anti-inflammatory drug, demarcation provider, epithelialization accelerator, and oedema reliever in traumatic and necrotic disorders as well as many infectious diseases [1-3]. In addition, TCAE can also be employed in treatment of clinical sign of foot and mouth disease and endometriosis [27,28]. However, TCAE can also be applied to the therapy of some cancer types [29]. Breast cancer is one of the most frequent cancers among 40-55-year-old women. Numerous risk factors, such as age, ionizing radiation, genetic makeup, and reproductive factors, cause breast cancer [30].
In this study, the apoptotic effect of TCAE was determined in the MCF-7 cell line (Figure 2). TCAE causes apoptosis in a mammary tumor model in dogs [28]. Moreover, recent data by Dizgah et al. reported that Theranekron lead to apoptotic death through activating caspase-3 in cancer cell lines [31]. Because there is limited data associated with the apoptotic effect of TCAE, we evaluated the effect of TCAE on certain cytokines. Antiapoptotic effects can be assessed via cytokines that play important roles in the physiology of cancer. In a coordinated manner, various cytokines, such as IFN, IL-6, IL-10 and TNF, play important roles in breast carcinogenesis [21].
The NF-κB level did not statistically differ between MCF-7 and MCF-7+TCAE groups (P>0.05, Table 1). However, the AUC value of NF-κB was decreased by TCAE in the MCF-7+TCAE group compared with the MCF-7 group (-7.02%). NF-κB is a transcription factor that mediates the synthesis of many molecules including inflammatory agents [13]. It plays dual roles as a tumor promoter or tumor growth inhibitor [12]. However, inhibition of NF-κB has a significant therapeutic effect against breast tumor progression [32]. The antiapoptotic activity is mediated by activation of TNF, while the activity of NF-κB is inhibited by IL-10 [9,13]. Because NF- κB is an intracellular substance and shows intracellular activity in the little amounts, it does not seem to excess the levels which make significant differences in the culture supernatant. Therefore, its level may not change by treatment with TCAE.
| Parameters | Group | 2 h | 4 h | 6 h | 8 h | 10 h | 24 h |
|---|---|---|---|---|---|---|---|
| TNFα (pg/mL) | MCF | 2.35 ± 1.32b | 0.83 ± 0.32b | 0.77 ± 0.35b | 1.28 ± 0.69b | 5.01 ± 3.87b | 17.27 ± 8.53a |
| MCF+TCAE | 0.81 ± 0.53a | 1.31 ± 0.58a | 0.38 ± 0.13a | 0.61 ± 0.32a | 0.61 ± 0.29a | 1.33 ± 0.026a | |
| IL-1β (pg/mL) | MCF | 9.90 ± 0.53a | 9.96 ± 0.18a | 10.46 ± 0.55a | 10.03 ± 0.78a | 10.24 ± 0.36a | 10.93 ± 0.88a |
| MCF+TCAE | 9.68 ± 0.23a | 10.31 ± 0.79a | 10.49 ± 0.37a | 9.68 ± 0.27a | 10.65 ± 0.57a | 12.30 ± 1.69a | |
| IL-6 (pg/mL) | MCF | 7.34 ± 1.80ab | 4.25 ± 1.62b | 8.43 ± 2.24ab | 12.83 ± 4.37a | 12.96 ± 1.82a | 3.77 ± 1.80b |
| MCF+TCAE | 2.63 ± 1.23a | 7.23 ± 2.55a | 10.53 ± 4.69a | 8.66 ± 2.44a | 1.73 ± 0.79a | 8.96 ± 3.07a | |
| IL-10 (pg/mL) | MCF | 4.05 ± 0.63a | 3.19 ± 0.61ab | 2.50 ± 0.46b | 2.71 ± 0.40ab | 3.36 ± 0.30ab | 3.68 ± 0.27ab |
| MCF+TCAE | 3.52 ± 0.72a | 5.31 ± 1.48a | 3.99 ± 0.89a | 3.52 ± 0.60a | 4.13 ± 0.59a | 4.33 ± 0.31a | |
| IFNγ (pg/mL) | MCF | 0.14 ± 0.05a | 0.23 ± 0.10a | 0.40 ± 0.12a | 0.28 ± 0.11a | 0.42 ± 0.24a | 0.45 ± 0.08a |
| MCF+TCAE | 0.55 ± 0.32a | 0.37 ± 0.08a | 0.56 ± 0.15a | 0.92 ± 0.20a | 0.56 ± 0.10a | 0.77 ± 0.25a | |
| TGFβ (pg/mL) | MCF | 2480 ± 125b | 2324 ± 92.3b | 2399 ± 82.0b | 2450 ± 139b | 2438 ± 75.3b | 2878 ± 154a |
| MCF+TCAE | 2369 ± 120a | 2399 ± 82.0a | 2254 ± 53.0a | 2426 ± 62.1a | 2495 ± 118a | 2411 ± 75.4a | |
| NF-κB (Abs) | MCF | 0.39 ± 0.01a | 0.47 ± 0.05a | 0.37 ± 0.01a | 0.41 ± 0.03a | 0.46 ± 0.03a | 0.43 ± 0.04a |
| MCF+TCAE | 0.41 ± 0.03a | 0.39 ± 0.03a | 0.39 ± 0.03a | 0.45 ± 0.03a | 0.39 ± 0.04a | 0.41 ± 0.03a |
Table 1. Effect of TCAE on TNFα, IL-1β, IL-6, IL-10, IFNγ, TGFβ and NFκB levels in MCF-7 cell line (Mean ± SE) breast carcinoma cell line, TCAE: Tarantula cubensis Alcoholic Extract; TNFα: Tumor Necrosis Factor Alpha; IL-1β: Interleukin-1 Beta; IL-6: Interleukin-6; IL-10: Interleukin-10; IFNγ: Interferon Gamma; TGFβ: Transforming Growth Factor Beta; NF-κB: Nuclear Factor Kappa B; Abs: Absorbance a, b: Different letters in the same row statistically are important (P<0.05).
In the present study, the TNFα level was highest at 24 h in the MCF-7 group (P<0.05), but did not change significantly in the MCF-7+TCAE group (P>0.05) (Table 1). In addition, the AUC value of TNFα was decreased in the MCF-7+TCAE group compared with the MCF-7 group (-87.33%). TNFα is highly expressed in breast cancer [15], and has an important role in the pathogenesis of cancer [33]. It has been reported that TNFα secreted by cancer cells extends the tumor cell life through induction of NF-κB-dependent antiapoptotic molecules and acts as tumor stimulants [9]. Because the serum TNFα level correlates with the tumor stage, this cytokine reflects the severity of staging for invasive breast cancer. Serum TNFα levels at stage I and II were higher than those in controls but not statistically significant. At stages III and IV, serum TNFα levels were significantly higher than those in the control group [34]. Anti-TNFα treatment is used in several types of cancer [11]. In addition, TNFα promotes chemotherapeutic resistance in cancer [35]. The augmentation of the TNFα level at 24 h may be caused by the normal cancer physiology in MCF-7 cells for migration and invasion.
The IL-1β level did not change significantly in MCF-7 and MCF-7+TCAE groups (P>0.05, Table 1). In addition, the AUC value of IL-1β increased in the MCF-7+TCAE group compared with the MCF-7 group (5.22%). In various types of cancer, invasiveness and metastasis of tumors have been associated with IL-1β levels [36,37]. A high level of IL-1β correlates with a high tumor grade in invasive breast carcinoma [38]. In breast cancer, a high level of IL-1Ra and low level of IL-1 in the tumor site correlate with a good prognosis [39]. In the present study, IL-6 and IL-10 levels showed significant fluctuations in the MCF-7 group (P<0.05), but did not change significantly in the MCF-7+TCAE group (P>0.05) (Table 1). In addition, the AUC value of IL-6 decreased in the MCF-7+TCAE group compared with the MCF-7 group (-34.40%). The AUC value of IL-10 increased in the MCF-7+TCAE group compared with the MCF-7 group (23.85%). The serum IL-6 level is high in breast cancer patients. IL-6 influences proliferation, angiogenesis and apoptosis in cancer [8,40]. In breast cancer patients, a low IL-6 level indicates a good response to therapy, whereas an increased IL-6 level indicates poor responses to chemoendocrine therapy [40]. IL-10 suppresses the production of IL-1β, TNFα, and IL-6 [21]. In addition, increased IL-10 might prevent tumor growth by suppressing IL-6 production. An inverse correlation exists between IL-6 and IL-10 levels in cancer patients [10]. Changes in IL-6 and IL-10 levels may be the result of normal cancer physiology in MCF-7 cells.
In this study, the TGFβ level was the highest at 24 h in the MCF-7 group (P<0.05), but it did not change significantly in the MCF-7+TCAE group (P>0.05) (Table 1). In addition, the AUC value of TGFβ decreased in the MCF-7+TCAE group compared with the MCF-7 group (-5.47%). TGFβ has immunosuppressive effects [13] and can play a role in metastasis [41]. It also increases tumor vascularity [42]. TGFβ suppresses the proliferation of cancer cells at early stages. However, TGFβ enhances proliferation of cancer cells, tumor progression, and invasiveness at later stages [22,23]. TGFβ participates in resistance to DNA-damaging chemotherapeutic agents in breast cancer cells [43]. Considering the requirement for immune system suppression in the development of cancer, it is assumed that TGFβ has been secreted by the tumor cells. An increased TGFβ level could arise from the effect of increasing levels of TNFα at the same time.
The IFNγ level did not change significantly in MCF-7 and MCF-7+TCAE groups (P>0.05, Table 1). The AUC value of IFNγ increased in the MCF-7+TCAE group compared with the MCF-7 group (70.38%). Breast tumor cells sensitizes to apoptosis by IFNγ [44]. Gooch et al. showed significant inhibition of MCF-7 cell growth by IFNγ [45]. The IFNγ level did not change significantly in both groups, but the AUC value of IFNγ was increased by TCAE.
In conclusion, the anticancer effect of TCAE may be exerted by disturbing cancer physiology or induction of apoptosis. TCAE can be used in the treatment of cancer. However, molecular studies should be performed to determine the anticancer mechanism of TCAE.
Acknowledgements
This study is supported by SUBAPK (14401119). A part of the abstract was presented at the “5th International Congress of Molecular Medicine” 20-22 May 2015, ?zmir, Turkey.
References
- Anonymous Richter Pharma, Theranekron D6. http://www.richter-pharma.at/product-theranekron-d6_301.html.
- Sertkaya H, Sindak N. Incidence and treatment with two different drug combinations of sheepsfootrot in birecik district of sanliurfa and its Villages. Vet CerDerg 2004; 10: 48-54.
- Sardari K, Kakhki EG, Mohri M. Evaluation of wound contraction and epithelialization after subcutaneous administration of Theranekron® in cows. Comp Clin Path 2007; 16: 197-200.
- Gultiken N, Vural M. The effect of Tarantula cubensis extract applied in pre and postoperative period of canine mammary tumours. JIVS 2007; 2: 13-23.
- Yardimci C, Yardimci B. Indolent ulcer in a cat. J FacVeter Med AnkUniv 2008; 55: 65-67.
- Icen H, Sekin S, Simsek A, Kochan A, Tunik S. The efficacy of tarantula cubensis extract (Theranekron) in treatment of canine oral papillomatozis. AJAVA 2011; 6: 744-749.
- Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med 2014; 11: 101-115.
- Culig Z. Cytokine disbalance in common human cancers. BiochimBiophysActa 2011; 1813: 308-314.
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 2007; 117: 1175-1183.
- Esquivel-Velazquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res 2015; 35: 1-16.
- Perkins ND. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol 2004; 14: 64-69.
- van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist 2006; 11: 397-408.
- Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res 2006; 4: 221-233.
- Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? ActaPharmacol Sin 2008; 29: 1275-1288.
- Hamed EA, Zakhary MM, Maximous DW. Apoptosis, angiogenesis, inflammation, and oxidative stress: basic interactions in patients with early and metastatic breast cancer. J Cancer Res ClinOncol 2012; 138: 999-1009.
- Waters JP, Pober JS, Bradley JR. Tumour necrosis factor and cancer. J Pathol 2013; 230: 241-248.
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357: 539-545.
- Apte RN, Krelin Y, Song X Dotan S, Recih E, Elkabets M, Carmi, Dvorkin T, White RM, Gayvoronsky L, Segal S, Voronov E. Effects of micro-environment- and malignant cell-derived interleukin 1 in carcinogenesis, tumour invasivenessand tumour-host interactions. Eur J Cancer 2006; 42: 751-759.
- Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med 2006; 4: 48.
- Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signalling pathway in targeted therapy for cancer. Cancer Treat Rev 2012; 38: 904-910.
- Hamidullah L, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res Treat 2012; 133: 11-21.
- Band AM, Laiho M. Crosstalk of TGF-γ and estrogen receptor signaling in breast cancer. J Mammary Gland BiolNeoplasia 2011; 16: 109-115.
- Joshi A, Cao D. TGF-beta signaling, tumor microenvironment and tumor progression: the butterfly effect. Front Biosci (Landmark Ed) 2010; 15: 180-194.
- Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. ProcNatlAcadSci USA 2012; 109: 16618-16623.
- Ruiz de Almodovar C, Lopez-Rivas A, Ruiz-Ruiz C. Interferon-gamma and TRAIL in human breast tumor cells. VitamHorm 2004; 67: 291-318.
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267: 1456-1462.
- Lotfollahzadeh S, Alizadeh MR, Mohri M, MokhberDezfouli MR. The therapeutic effect of Tarentulacubensis extract (Theranekron(R)) in foot-and-mouth disease in cattle: a randomised trial in an endemic setting. Homeopathy 2012; 101: 159-64.
- Dolapcioglu K, Dogruer G, Ozsoy S, Ergun Y, Ciftci S, SoyluKarapinar O, Aslan E. Theranekron for treatment of endometriosis in a rat model compared with medroxy progesterone acetate and leuprolide acetate. Eur J ObstetGynecolReprodBiol 2013; 170: 206-210.
- Gultiken N, Guvenc T, Kaya D, Agaoglu A.R, Ay SS, Kucukaslan I, Emre B, Findik M, Schäfer-Somi S, Aslan S. Tarantula cubensis extract alters the degree of apoptosis and mitosis in canine mammary adenocarcinomas. J Vet Sci 2015; 16: 213-219.
- Srinivasan V, Spence DW, Pandi-Perumal SR, Trakht I, Cardinali DP. Therapeutic actions of melatonin in cancer: possible mechanisms. Integr Cancer Ther 2008; 7: 189-203.
- Dizgah AG, Nami B, Amirmozafari N. Tarantula cubensis venom (Theranekron®) selectively destroys human cancer cells via activating caspase-3-mediated apoptosis. ActaMedicaInt 2017; 4: 73-79.
- Connelly L, Barham W, Onishko HM, Sherrill T, Chodosh LA, Blackwell TS, Yull FE. Inhibition of NF-kappa B activity in mammary epithelium increases tumor latency and decreases tumor burden. Oncogene 2011; 30: 1402-1412.
- Yang Y, Feng R, Bi S, Xu Y. TNF-alpha polymorphisms and breast cancer. Breast Cancer Res Treat 2011; 129: 513-519.
- Sheen-Chen SM, Chen WJ, Eng HL, Chou FF. Serum concentration of tumor necrosis factor in patients with breast cancer. Breast Cancer Res Treat 1997; 43: 211-215.
- Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets 2010; 11: 1133-1146.
- Song X, Voronov E, Dvorkin T, Fima E, Cagnano E. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J Immunol 2003; 171: 6448-6456.
- Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1 promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer 2012; 11: 87.
- Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A. Expression of interleukin-1beta in human breast carcinoma. Cancer 1997; 80: 421-434.
- Perrier S, Caldefie-Chézet F, Vasson MP. IL-1 family in breast cancer: potential interplay with leptin and other adipocytokines. FEBS Lett 2009; 583: 259-265.
- Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res 1999; 19: 1427-1432.
- Paradkar PH, Joshi JV, Mertia PN, Agashe SV, Vaidya RA. Role of cytokines in genesis, progression and prognosis of cervical cancer. Asian Pac J Cancer Prev 2014; 15: 3851-3864.
- Ito N, Kawata S, Tamura S, Shirai Y, Kiso S, Tsushima H, Matsuzawa Y. Positive correlation of plasma transforming growth factor-beta 1 levels with tumor vascularity in hepatocellularcarcinoma. Cancer Lett 1995; 89: 45-48.
- Yu Y, Wang Y, Ren X, Tsuyada A, Li A, Liu LJ, Wang SE. Context-dependent bidirectional regulation of the MutS homolog 2 by transforming growth factor ß contributes to chemoresistance in breast cancer cells. Mol Cancer Res 2010; 8: 1633-1642.
- Ruiz-Ruiz C, Muñoz-Pinedo C, Lopez-Rivas A. Interferon-gamma treatment elevates caspase-8 expression and sensitizes human breast tumor cells to a death receptor-induced mitochondria-operated apoptotic program. Cancer Res 2000; 60: 5673-5680.
- Gooch JL, Herrera RE, Yee D. The role of p21 in interferon gamma-mediated growth inhibition of human breast cancer cells. Cell Growth Differ 2000; 11: 335-342.