- Biomedical Research (2012) Volume 23, Issue 4
Age wise distribution of high risk Human Papillomavirus in Northern Indian women.
Narotam Sharma, Veena Sharma1, Prem Raj Singh2, R.S. Kushwaha, Satish Chandra Nautiyal, Shivani Sailwal, Rajesh K Singh, Tariq Masood, Pankaj Mishra, R.K Singh*.Molecular Research Laboratory, Departments of Biochemistry and Community Medicine, Shri Guru Ram Rai Institute of Medical & Health Sciences, Patel Nagar, Dehradun-248001(Uttarakhand)
1Department of Bioscience and Biotechnology, Banasthali University, Banasthali – 304022, (Rajasthan)
2Auroprobe Laboratory, Muradnagar, Ghaziabad- Uttar Pradesh
- Corresponding Author:
- R.K. Singh
Molecular Research Laboratory
Department of Biochemistry
SGRRIM&HS, Patel Nagar
Dehradun (U.K), India
Accepted date: September 04 2012
Abstract
Human papillomavirus (HPV) testing was introduced to compensate the poor sensitivity and specificity of the pap smear cytology often used as a diagnostic tool for borderline precancerous lesions. Digene Hybrid Capture Assay 2 (HCA-2) is the only approved test by the U.S. Food and Drug Administration (FDA) for evaluation and confirmation of the cytologically borderline suspected cases. 361 cervical specimens were collected for the high risk HPV analysis. Forty one cervical samples were positive. Age wise distribution showed highest prevalence in the 15-35 age groups which decreased in subsequent years. Present observations are in agreement with similar studies reported from other parts of the world including Southern India
Keywords
Hybrid capture assay; pap smear; Human Papillomavirus; DNA-RNA hybrids.
Introduction
Cancer of the cervix is the second most common cancer among women worldwide. India has a population of 366.58 million women ages 15 years and older who are at risk of developing cervical cancer. Current estimates indicate that every year 134420 women are diagnosed with cervical cancer and 72825 die from the disease. Cervical cancer ranks as the 1st most frequent cancer among women in India, and the 1st most frequent cancer among women between 15 and 44 years of age [1]. The persistent infection by specific high- risk human papillomavirus (HR-HPV) is essential for the progression of cervical lesions and women who are infected with HR-HPVs are likely to develop cancer. HR-HPV detection holds the potential to diagnose the women in early stages before the progression of the disease and provides the base to be used as a tool to identify the women at the risk of subsequent development of cervical cancer. For early treatment and management of cervical cancer, generalized and genital warts identification of HR-HPV is necessary. Nearly all the invasive cervical cancer and high grade intraepithelial neoplasias are linked with HR-HPV types, which are now universally accepted. It develops following progression of uncleared HPV infection to high grade and eventually to invasive disease. Cancerous HPV types are associated with cervical cancer, and non-cancerous HPV types are associated with warts of the genital areas and low grade disease of the cervix [2]. Various studies have demonstrated that more than 98% of invasive cervical cancers harbor HPV type 16 (HPV-16) and HPV-18 [3]. More than 100 genotypes within the family of HPV are known which vary in their tissue tropism and oncogenic potential. HPV types are defined on the basis of homology of the viral genome [4]. The risk of acquiring anogenital HPV infection is associated mainly with early sexual experience, number of lifetime sexual partners, and sexual contact with highly promiscuous partners [4,5]. Persistent infection with HPV has been identified as the most important cause of cervical cancer [6]. Supervision by cytological observation has raised worry that some cases of high grade disease might escape findings because of absence or inadequate understanding and those women might practice anxiety over a prolonged period [7]. Diagnosis of cervical disease, indicating the presence of abnormal cervical epithelial cells, is usually obtained by colposcopic examination by Papanicolaou stain smear [8]. This has been the method of choice since the 1950's, proving valuable tool for mass screening and enabling detective lesion early enough to be effectively treated. The Pap smear, however, has limited sensitivity detecting cancer precursors, giving a false negative rate ranging from 20 to 30 % [9]. In developing nations there is a lack of effective screening programs for cervical cancer [10]. It has been reported that no clinically significant reduction in the incidence of cervical cancer has occurred during the past three decades [11,12]. There are few reports available regarding age wise distribution of HR-HPV from Northern part of the country. The present study was planned to detect age wise distribution of HR-HPV through DNARNA hybridization technology in local population.
Material sand Methods
Three hundred and sixty one cervical specimens from different hospitals in Delhi / NCR region and SMI Hospital, Patel Nagar, Dehradun were collected for the present study. The cervical cytobrush was used for the collection of samples from cervix and were transported in virus transport media (Digene Diag, Md). Hybrid capture assay II (HCA II) from Digene Diagnostics (Silver Spring, Md.) was used for the detection of HR-HPV. Following standardized procedure was used for HR-HPV detection (Digene Diagnostics, Silver Spring, Md.): An Invitro Nucleic Acid Hybridization Assay with signal amplification using microplate chemiluminiscence for the qualitative detection of HPV types 16,18,31,33,35,39,45,51,52,56,58,59, 68 was used. The Hybrid Capture 2 HR-HPV DNA Test using Hybrid Capture 2 technology is a nucleic acid hybridization assay with signal amplification that utilizes microplate chemiluminescent detection. Specimens containing the target DNA hybridize with a specific HPV RNA probe cocktail. The resultant RNA: DNA hybrids are captured onto the surface of a microplate well coated with antibodies specific for RNA: DNA hybrids. Immobilized hybrids are then reacted with alkaline phosphatase conjugated antibodies specific for the RNA: DNA hybrids, and detected with a chemiluminescent substrate. Several alkaline phosphatase molecules are conjugated to each antibody. Multiple conjugated antibodies bind to each captured hybrid resulting in substantial signal amplification. As the substrate is cleaved by the bound alkaline phosphatase, light is emitted that is measured as relative light units (RLUs) on a luminometer. The intensity of the light emitted denotes the presence or absence of target DNA in the specimen. An RLU measurement equal to or greater than the Cutoff Value (CO) indicates the presence of high-risk HPV DNA sequences in the specimen. An RLU measurement less than the Cutoff Value indicates the absence of the specific high-risk HPV DNA sequences tested or HPV DNA levels below the detection limit of the assay. All the, specimens were treated with denaturing solution containing NaOH to denature the ds circular DNA of Human Papilloma Virus. The liberated single stranded DNA was hybridized in solution with a RNA probe cocktail for thirteen high risk groups of HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68). Each reaction mixture, containing any number of RNA: DNA hybrids that formed, was transferred to a microtitre plate containing capture tube coated with anti RNA: DNA hybrid antibodies; consequently, immobilizing them. Nonreactive material was removed by washing, and a dioxetane – based chemiluminescent compound, Lumi–phos 530, was added as a substrate for alkaline phosphatase. The light produced by the ensuing reaction was measured by Luminometer. Light measurements were expressed as Relative light units (RLU). As a negative control, sonicated herring sperm DNA in Digene transport media (100 mg/ml) was used. Triplicate specimens of HPV 16 or HPV 11 DNA at 10 pg / ml served as a positive control for high – risk probes. All RLU measurements for specimens were divided by the mean RLU of the three appropriate positive controls (PCs) to give a ratio of specimen RLU/PC. A ratio of 1.0 was regarded as negative Since the amount of light produced by the hybrid capture assay is theoretically proportional to the amount of target HPV DNA, HCA II can be analyzed as a quantitative method.
Results
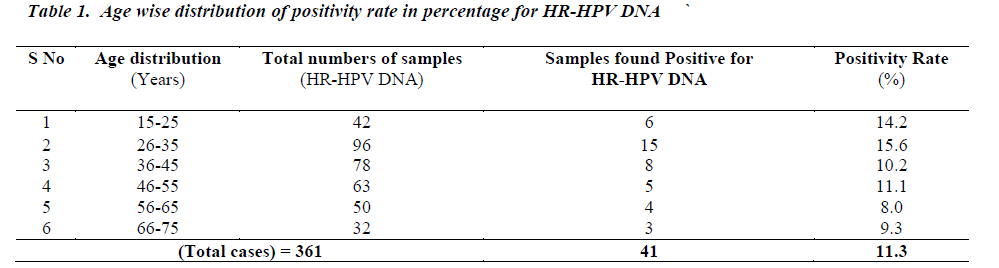
High risk Human Papillomavirus was detected in 41 cases (11.3%). In terms of age wise distribution of HR-HPV, it was observed that positivity rate for the age groups 15- 25,26-35,36-45, 46-55, 56-65 , 66-75 was 14.2 %, 15.6 %, 10.2 %, 11.1 %, 8.0 % and 9.3% respectively.
Discussion
HPV is considered as a public health problem for its role as a significant factor in pathogenesis of various types of associated cancers. From various studies, it has been reported that HPV infection is increasing every year which could be due to uninhibited sexual encounters resulting into increased incidence of HPV infection [13]. Present observations showed the incidence of HPV infection in younger age group as compared to the middle and older age group. The age group with most incidence of HPV infection is 15 to 35 years. This is the age which is most sexually active [14]. There is a gradual decrease in incidence of HPV infection in later years. The number of infection is increasing due to many factors and most prominent is of preventive ignorance. Even though the number of educated population is increasing day by day in developing nations like India, the awareness level of HPV is nonexistent [15]. People are aware of many sexually transmitted diseases like AIDS for which there are several programmes promoted by different agencies around the country. But there are no coherent programmes to extend awareness about HPV which leave women totally unaware of this infection. As a result there is no timely intervention of the infection which later on develops into cancer. Early detection can greatly reduce cervical cancer deaths if the infection is diagnosed in initial stages. So, a rational policy about the awareness of HPV infection is absolutely necessary to stop the increase of the cervical cancer by detecting the HR-HPV at early stages. Such knowledge and diagnostic approach will be helpful in management of concerned patients [16]. Our results confirmed that HR-HPV infection is mainly associated with early sexual experience, multiple sexual partners, unhygienic conditions, lack of awareness programmes and traditional family relations. In India many findings from rural areas of southern parts have showed higher incidence of HPV infection in similar age groups. However, present observations showed the prevalence rate to be 11.3% which could probably be due to better socioeconomic conditions and preventive measures. The hybrid capture test has good reliability and accuracy, although room for improvement remains. Further studies on larger population are needed to detect the specific types of HRHPV for better management of cervical cancer.
Acknowledgement
The authors are thankful to the authorities of Banasthali University and Auroprobe Laboratory for providing support to the study.
References
- WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre). Human Papillomavirus and Related Cancers in India. Summary Report, 2010.
- Dianne J. Marais,Debbie Constant, Bruce Allan,Henri Carrara, Margaret Hoffman, Samuel Shapiro, Chelsea Morroni, and Anna-Lise Williamson. Cervical Human Papillomavirus (HPV) Infection and HPV Type 16 Antibodies in South African Women. J Clin Microbiol 2008; 46: 732-739.
- Garland SM. Human papillomavirus update with a particular focus on cervical disease. Pathology 2002; 34: 213-224.
- Kjaer SK, Chackerian B, van den Brule AJ, Svare EI, Paull G, Walbomers JM. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 2001; 10(2): 101- 106.
- Weaver BA. Epidemiology and natural history of genital human papillomavirus infection. J Am Osteopath Assoc 2006; 106: 2-8.
- Moscicki AB. HPV infections in adolescents. Dis Markers 2007; 23(4): 229-234.
- Schmitt M, Bravo IG, Snijders PJ, Gissmann L, Pawlita M, Waterboer T: Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol 2006; 44: 504- 512.
- Clavel C, Masure M, Putaud I, Thomas K, Bory JP, Gabriel R. Hybrid capture II, a new sensitive test for human papillomavirus detection. Comparison with hybrid capture I and PCR results in cervical lesions. J Clin Pathol 1998; 51(10): 737-740.
- Yamada T, Manos MM, Peto J, Greer CE, Munoz N, Bosch FX. Human papillomavirus type 16 sequence variation in cervical cancers: a worldwide perspective. J Virol 1997; 71(3): 2463-2472.
- Wright TC Jr, Ellerbrock TV, Chiasson MA, Van Devanter N, Sun XW. Cervical intraepithelial neoplasia in women infected with human immunodeficiency virus: prevalence, risk factors, and validity of Papanicolaou smears. New York Cervical Disease Study. Obstet Gynecol 1994; 84: 591-597.
- Sankaranarayanan R, Budukh A, Rajkumar R. Effective screening programs for cervical cancer in low- and middle income developing countries. Bull World Health Organ 2001; 79: 954-962.
- Ferlay J, Parkin DM, Pisani P. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. IARC Cancer Base no. 5, version 2.0. Lyon, France: IARC Press, 2004.
- Cervix cancer screening. Vol. 10. IARC handbooks on cancer prevention. Lyon, France: IARC Press, 2005.
- Huang SL, Chao A, Hsueh S, Chao FY, Huang CC, Yang JE, Lin CY, Yan CC, Chou HH, Huang KG: Comparison between the Hybrid Capture II Test and an SPF1/GP6+ PCR-based assay for detection of human papillomavirus DNA in cervical swab samples. J Clin Microbiol 2006; 44(5): 1733-1739.
- Wright TC Jr, Cox JT, Massad LS, et al.2001 consensus guidelines for the management of women with cer vical cytological abnormalities. J Am Med Assoc. 2002; 287: 2120-2129.
- Ronco G, Segnan N, Giorgi-Rossi P, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006; 98: 765-774.