Case Report - Archives of General Internal Medicine (2018) Volume 2, Issue 2
Acute Transverse Myelitis: A Rare Presentation of Dengue Fever.
Bijaya Mohanty*, Sameer Mehta and Asif Ahmed
Department of Medicine & Critical Care Unit, Tata Main Hospital, Jamshedpur, India
- *Corresponding Author:
- Bijaya Mohanty
Department of Medicine
Tata Main Hospital
Jamshedpur, India
E-mail: bijayamohantytmh@gmail.com
Accepted on March 16, 2018
Citation: Mohanty B, Mehta S, Ahmed A. Acute transverse myelitis: A rare presentation of dengue fever. Arch Gen Intern Med. 2018;2(2):23-26.
Abstract
Spinal cord involvement in the form of acute transverse myelitis is an uncommon manifestation of dengue virus infection. Only few cases have been reported so far in the literature. Here we are reporting a 32 year old man who developed features of acute transverse myelitis six days after the onset of dengue fever. His magnetic resonance imaging of spinal cord showed segmental T2 hyper intensity from D4 to D6 levels involving predominantly the central component. He was treated with intravenous methyl prednisolone, Intravenous Immunoglobulin (IV IG) and physiotherapy. He improved substantially after four months & after seven months he recovered completely. He is able to walk normally & do his activities of daily living independently. Though neurological manifestations are reported in dengue fever, involvement of spinal cord in the form of transverse myelitis is extremely rare. Isolated reports are the tip of the iceberg. Its incidence may rise in the near future demanding increased awareness among health care providers.
Keywords
Acute transverse myelitis, Paraplegia, Dengue fever, Flavivirus Methylprednisolone, Immunoglobulin
Introduction
Dengue fever is a mosquito-borne disease caused by dengue virus. There is a rising trend in the incidence of dengue fever all over the world especially in tropical countries over the past few years. Dengue cases in India are also on a sharp rise over past few years to the tune of 28,292 cases in 2010 to 1,11,880 cases in 2016 [1,2] posing a challenge to health care providers. We are all aware of classic dengue fever, DHF (Dengue haemorrhagic fever) & DSS (Dengue shock syndrome). Recently it has been observed that the clinical presentations of dengue fever is changing its horizon and neurological manifestations are more commonly observed & reported involving both central and peripheral nervous system. Patient can present with features of encephalitis, meningitis, stroke (both haemorrhagic and ischemic), hypokalemic paralysis, encephalopathy, seizures, mono-neuropathy, polyneuropathy, and Guillain-Barre or Miller-Fisher syndromes [3-6]. Dengue virus infection involving spinal cord is extremely rare. Only few cases of transverse myelitis in patients with dengue fever have been reported so far [7-10]. Here we are reporting a young man who developed transverse myelitis after six days of onset of dengue fever.
Case Summary
A 32 year old male attended the emergency department with complains of fever along with dry cough for six days followed by dribbling ´ retention of urine for which he was catheterized. Patient denies any history of trauma, recent vaccination or radiation. On examination he was febrile with a temperature of 100 degree Farenheit. There was no pallor, icterus, cyanosis & lymphadenopathy. His vital parameters were well within normal limits. Systemic examination also did not reveal any abnormality. Basic investigations including complete blood picture, liver function test were within normal limits. His haematocreit level was 38% & platelet count was 96000 per cubic mm. There was no evidence of bleeding from any site. Dengue NS1 antigen was positive. He was treated symptomatically. Over-night he developed loss of control over both lower limbs and absent sensation from the chest below. He was hemodynamically stable. A detailed central nervous system examination was done. He was well oriented to time, place and person. There were no mood or psychotic symptoms. There was no history of blurring of vision, diplopia, facial numbness, ataxia, dysphagia or upper limb weakness. There was no prior history of polyarthralgia, malar rash, uveitis or oral ulcer. There was no cranial nerve involvement. Motor system examination revealed normal bulk in all four limbs. There was hypotonia in both lower limbs. Power was grade 1/5 in both lower limbs & grade 5/5 in both upper limbs. All modalities of sensation were diminished below D8 level. There was no involuntary movement. Perianal sensations were absent but deep anal pressure & bulbo-cavernosus reflex were present. Distal tendon reflexes were absent in both lower limbs (knee & ankle) & normal in upper limbs (biceps, triceps & supinator). Superficial abdominal reflexes were present in upper quadrants & absent in lower quadrants. Plantar reflexes were bilaterally mute. Cerebellar signs were absent.
His haemoglobin was 12.5 gm %, total leucocyte count was 7500/ cumm. Platelet count varied between a minimum of 1,04,000 to maximum value of 2,59,000 per cubic mm during his hospital stay. Liver function test revealed normal bilirubin level with a mild rise in enzymes. Renal function test, serum electrolytes & serum protein level were within normal range. Blood & urine cultures were sterile. Blood for ANA was negative. Viral markers like HBSAg, HCV, and HIV were negative. TSH was within normal limits. CSF analysis showed total cell of 40 with 85% lymphocytes. ADA was 1.4 & there was a mild rise in CSF protein (69.5 mg %). CT & MRI Brain was normal. MRI spine was suggestive of myelitis dorsal spinal cord D7-D8 level probably of inflammatory origin.
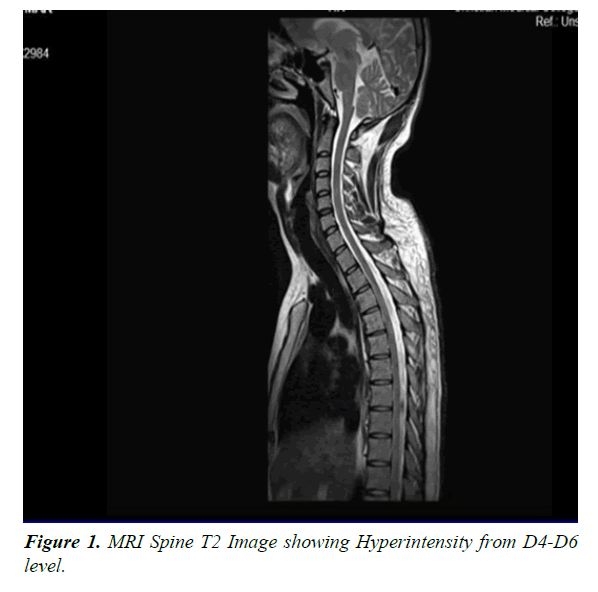
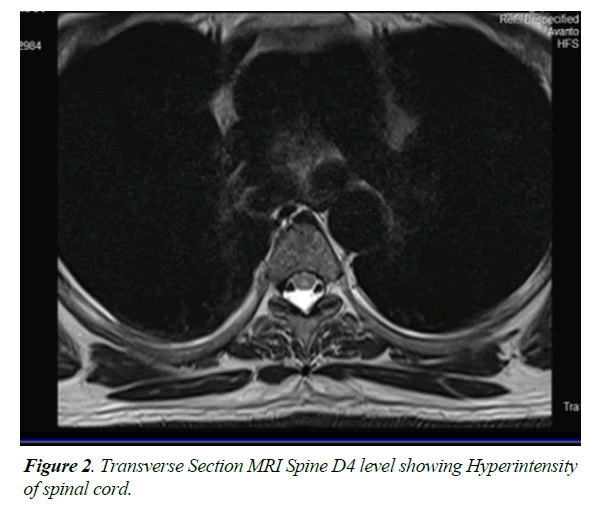
He was diagnosed as acute transverse myelitis following dengue fever & was treated with injection methylprednisolone one gram intravenously daily for one week followed by oral tablets along with other supportive care including physiotherapy. Injection enoxaparin 0.4 ml subcutaneously once daily was also given for DVT prophylaxis. Though he was hemodynamically stable there was no change in neurological status. Around two weeks after the steroids he noticed gradual however minimal improvement in his right hip & ankle. He was discharged & referred to a higher centre. He was wheel chair bound & dependent for all activities of daily living. He was on a continuous bladder drain & is incontinent for bowel needs. His glycosylated haemoglobin was 6%, Vitamin B12 & folic acid were within normal limits. Serum Vitamin D was low (9.8 ngm/ml), Rheumatoid factor was less than 10.4 IU/ml. CSF culture was negative for bacteria. Blood culture was sterile. Fungal culture of CSF was negative for Cryptococci. XPERT TB PCR was negative for mycobacterium tuberculosis. Mycobacterial culture (MGIT Automation) was also negative. PCR for multiple viruses in CSF was negative. A Cytospin smear of CSF was also negative for malignant cells. Special antibody tests like Anti SSB, Anti SSA by Elisa were negative. Anti-AQUAPORIN -4 by IIFT (Indirect immune florescence test) was also negative. His MRI spine was repeated at the higher centre which showed segmental T2 hyperintensity of the spinal cord from D4 to D6 levels involving predominantly the central component (Figures 1 and 2). There was no evidence of cord expansion or contrast enhancement or involvement of the other segments of the spinal cord.
So considering para infectious viral (Dengue) myelitis he was given adjuvant IVIG 2 gram/kg (total 120 gm) over 5 days.
But there was no appreciable improvement in motor power. He was started on occupational & physiotherapy. His stay was complicated by catheter induced urinary tract infection & pressure sore which was taken care of with antibiotics & regular dressing. His urodynamic studies showed a poorly compliant bladder with a lower motor neuron type of bladder. A rectal ultrasound showed an open bladder neck. He was taught to use bisacodyl suppositories & digital evacuation for bowel management.
In physiotherapy strengthening exercises were given for the key group of muscles. He was found to have gradual development of tone & improvement in voluntary control of both lower limbs. Now after seven months he is able to walk without any support. His muscle power has improved to grade 5/5 in both lower limbs. He is also independent in all activities of daily living. He is being followed up in medicine unit regularly.
Discussion
Dengue fever is a mosquito-borne viral disease which has reached alarming proportions in the past few years. It is Endemic in over 100 countries [11]. 40% of people all over the world live in countries where dengue is endemic. Annually 390 million infections and 5,00,000 dengue haemmorhagic fever cases have been identified. Dengue cases in India are also on a sharp rise over past few years to the tune of 28,292 cases in 2010 to 1,11,880 cases in 2016 posing not only a challenge to clinicians but also a threat to the community at large. Dengue epidemics mostly occur in tropical and subtropical countries and pose a serious public health problem [12,13]. Dengue virus is an arthropod borne virus of genus flavivirus belonging to family flaviviridae. It is a single stranded RNA virus. There are four genetically related but antigenically distinct DEN serotypes (DENV-1, DENV-2, DENV-3, DENV-4) all of which are prevalent in India. Clinical presentation of Dengue virus infection vary from subclinical to fatal manifestations such as dengue hemorrhagic fever and dengue shock syndrome. But this classic dengue presentation has expanded its horizon by involving various organ systems recently & is named as expanded dengue syndrome by WHO 2016. Central & peripheral nervous system involvement in dengue infection have been reported in various studies. [3-6,14-19]. But spinal cord involvement in the form of transverse myelitis is extremely rare. There have been only few previously reported cases of transverse myelitis in association with dengue infection [7-10]. Solomon and colleagues reported two cases of transverse myelitis out of 21 patients of dengue fever with neurological involvement [20]. Ferreira et al. also described two ATM (Acute transverse myelitis) cases out of 41 cases [21] and Sohler et al. too reported two cases of TM in a series of 13 patients [22]. The duration between the onset of infection and the development of acute transverse myelitis varied from 2 to 16 days in the previously reported cases. In our case he developed features of transverse myelitis after 6 days. The exact pathogenesis of neurological manifestations of dengue viral infection is not known. However there are several postulations. Most important is either the neurotropic effect of the virus or the immune mediated injury or both. When the neurologic symptoms develop in peri-infectious period it is attributed to direct viral invasion of the nervous tissue. Delayed appearance of neurologic disorders usually in post infectious phase are considered to be due to immunologically mediated neural injury [10]. Isolation of the dengue virus antigen from CSF and spinal cord tissue in the cases of transverse myelitis immediately following dengue infection supports the theory of neurotropic effect of the virus [8,9,11].
The term myelitis is defined as inflammation of the spinal cord which damages the nerve fibers & causes them to lose their myelin sheath leading to decreased electrical conductivity in the central nervous system. In transverse myelitis the inflammation extends across the entire width of the spinal cord [12]. When the inflammation affects part of the width of the spinal cord is referred to as partial myelitis. Usually it involves one segment of the cord [12]. When the lesion extends over three or more vertebral segments it is known as longitudinally extensive transverse myelitis (LETM) or long segment transverse myelitis which happened in our case. It can affect any segment of the spinal cord but thoracic segments are most commonly involved which is there in our case too. Though the etiology of transverse myelitis is manifold usually it is caused by various infections, immune system disorders and other disorders that may damage or destroy myelin. It can occur in the post or para infectious phase of several bacterial & viral infections. The viruses which cause transverse myelitis are CMV, herpes simplex, herpes zoster, Epstein-Barr virus, HIV [23,24] & rarely Dengue virus. Transverse myelitis can occur after post vaccination. Even idiopathic presentation is not uncommon. Aquaporin-4 autoantibody should be done to rule out associated neuromyelitis optica & multiple sclerosis in all cases of transverse myelitis. The diagnostic criteria are based on the Transverse Myelitis Consortium Working Group 2002 which includes motor, sensory or autonomic dysfunction attributable to spinal cord. In transverse myelitis usually signs and symptoms occur on both sides of the body which msy not necessarily symmetrical in nature. There is always a clearly defined sensory level (D8 level in our case). Signs of inflammation are shown as pleocytosis of the cerebrospinal fluid, or elevated immunoglobulin G, or evidence of inflammation on gadolinium-enhanced MRI spine. All these features are present in our case. Post-infectious myelitis, unlike infectious myelitis, may be associated with a higher frequency of normal CSF [25-28]. Diagnosis is confirmed by MRI of whole spine. However MRI of brain has to be done in all cases which will help to rule out other underlying causes especially multiple sclerosis. In our case MRI Brain was normal. Point to be noted that a normal MRI of spine also does not rule out or invalidate the clinical diagnosis of transverse spinal cord syndrome [29]. Leao et al. and Seet et al. reported two cases of TM with normal MRI [30,31]. A repeat MRI spine may be advised to delineate structural damage of the cord in these cases. Blood tests for HIV infection, vitamin B12 deficiency and for the presence of autoantibodies like anti-aquaporin-4, anti-myelin & paraneoplastic antibodies should be done. Our patient was negative for anti-aquaporin 4 antibody.
The main stay of treatment is to reduce the spinal cord inflammation & manage the symptoms. The cause of transverse myelitis needs to be addressed. Initial treatment includes intravenous corticosteroids which not only reduce swelling and inflammation in the spine but also reduce activity of immune system. Usually methylprednisolone or dexamethasone is administered intravenously for a period of five to seven days. Intravenous corticosteroids also helps in reducing subsequent attacks of transverse myelitis. People who don't respond well to intravenous steroids are treated with plasmapheresis. Intravenous immunoglobulin (IVIG) is another modality of treatment which is thought to stabilise the deranged immune system. Antiviral medications help those individuals who have a viral infection of the spinal cord. Aggressive physiotherapy is a must for all patients. Our patient was treated with intravenous corticosteroids, IVIG & physiotherapy. In most patients with transverse myelitis the recovery is slow with most recovery taking place within the first 3 months after the attack. Our patient improved within three months & after seven months his power in lower limbs improved to grade 5/5. He is able to walk normally & do his activities of daily living independently. In some cases, recovery may continue for up to 2 years and even longer. However, if there is no improvement within the first 3 to 6 months, complete recovery is unlikely. Prompt & aggressive treatment approach with corticosteroids, IVIG & physiotherapy have been shown to improve outcomes which was there in our case too.
Conclusion
Rising trend of dengue epidemics in both tropical & sub-tropical countries & increasing neurological manifestation poses a challange to the health care providers & community at large. Isolated occurence of acute transverse myelitis in dengue fever is the tip of the iceberg. Its incidence may rise in the near future demanding increased awareness which will prevent under reporting. Once diagnosed treatment on war foot basis will help the patient gaining complete recovery.
References
- WHO Dengue and severe dengue. Fact sheet. April 2017.
- Ghan Shyam Pangtey, Anupam Prakash, Yash Pal Munjal, et al. Role of Carica papaya Leaf Extract for Dengue Associated Thrombocytopenia. J Assoc Physicians India. 2016;64:11-2.
- Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. Lancet. 2000;355:1053-9.
- Patey O, Ollivaud L, Breuil J, et al. Unusual neurologic manifestations occurring during dengue fever infection. Am J Trop Med Hyg. 1993;48:793-802.
- Gaultier C, Angibaud G, Laille M, et al. Probable Miller-Fisher syndrome during dengue fever type 2. Rev Neurol (Paris). 2000;156(2):169-71.
- Jackson ST, Mullings A, Bennetts F, et al. Dengue infection in patients presenting with neurological manifestations in a dengue endemic population. West Indian Med J. 2008;57(4):373-6.
- Renganathan A, Keong W, Tin Tan C. Transverse myelitis in association with Dengue infection. Neurol J Southeast Asia. 1996;1(1):61-3.
- Leao RN, OiKawa T, Rosa ES, et al. Isolation of dengue 2 virus from a patient with central nervous system involvement (transverse myelitis) Rev Soc Bras Med Trop. 2002;35(4):401-4.
- Kunishige M, Mitsui T, Tan BH, et al. Preferential gray mater involvement in dengue myelitis. Neurology. 2004;63(10):1980-1.
- Seet RCS, Lim ECH, Wilder-Smith EPV. Acute transverse myelitis following dengue virus infection. J Clin Virol. 2006;35(3):310-2.
- Lum LC, Lam SK. Dengue encephalitis: a true entity. Am J Trop Med Hyg. 1996;54(3):256-9.
- Péres JGR, Clark GC, Gubler DJ, et al. Dengue and Dengue hemorrhagic fever. Lancet. 1998;352:971-7.
- Guzmán GM, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33-42.
- Carod-Artal FJ, Wichmann O, Farrar J, et al. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12:906-19.
- Hommel D, Talarmin A, Delbel V, et al. Dengue encephalitis in French Guiana. Res Virol. 1998;149: 235-8.
- Palma-da Cunha-Matta A, Soares-Moreno SA, Cardoso-de Almeida A, et al. Complicaciones neurológicas de la infección por el vírus del dengue. Rev Neurol. 2004;39:233-237.
- Miranda de Sousa A, Puccioni-Sohler M, Dias Borges A, et al. Post-Dengue neuromyelitis optica: case report of a Japanese Brazilian Child. J Infect Chemother. 2006;12:396-8.
- Yamamoto Y, Tokasaki T, Yamada K, et al. Acute disseminated encephalomyelitis following dengue fever. J Infect Chemother. 2002;8:175-7.
- Sundaram C, Uppin SG, Dakshinamurthy KV, et al. Acute disseminated encephalomyelitis following dengue hemorrhagic fever. Neurol India. 2010;58:599-601.
- Salomon T, Dung NM, Vaughn DW, et al. Neurologic manisfestation of dengue infection. Lancet. 2000;355:1053-9.
- Ferreira MLB, Cavalcanti CG, Coelho CA, et al. Manifestações neurológicas de dengue estudo de 41 casos. Arq Neuropsiquiatr. 2005;63:488-93.
- Puccioni-Sohler M, Soares CN, Papaiz-Alvarenga R, et al. Neurologic dengue manifestations associated with intratecal especific immune response. Neurology. 2009;73:1413-7.
- Tranverse Myelitis Consortion Working Group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499-505.
- Krishnan C, Kaplin AL, Deshpande DM, et al. Transverse Myelitis: pathogenesis, diagnosis and treatment. Front Biosci. 2004;9:1483-99.
- Miranda de Sousa A, Puccioni-Sohler M, Dias Borges A, et al. Post-Dengue neuromyelitis optica: case report of a Japanese Brazilian Child. J Infect Chemother. 2006;12:396-8.
- Tranverse Myelitis Consortion Working Group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499-505.
- Krishnan C, Kaplin AL, Deshpande DM, et al. Transverse Myelitis: pathogenesis, diagnosis and treatment. Front Biosci. 2004;9:1483-99.
- Jakob A, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Semin Neurol. 2008;28:105-20.
- Krishnan C, Kaplin AL, Deshpande DM, et al. Transverse Myelitis: pathogenesis, diagnosis and treatment. Front Biosci. 2004;9:1483-99.
- Leão RNQ, Oikawa T, Rosa EST, et al. Isolation of dengue 2 virus from a patient with central nervous system involvement (tranverse myelitis). Rev Soc Bras Med Trop. 2002;35:401-4.
- Seet RC, Lim EC, Wilder-Smith EP. Acute transverse myelitis following dengue vírus infection. J Clin Virol. 2006;35:310-2.