Research Article - Journal of Physical Therapy and Sports Medicine (2023) Volume 7, Issue 4
Acute Effects of a Novel, Calf-Only External Pneumatic Compression Device on Measures of Lower-Limb Blood Flow, Blood Volume and Tissue Oxygenation
Gabriel K. Morales1, Jessica T. Bui1, Joseph M. Steinhauer1, Rachel L. Hickman1,2, Jeffrey S. Martin1*
1Department of Basic Medical Sciences, DeBusk College of Osteopathic Medicine, Lincoln Memorial University, Knoxville TN, USA
2Department of Public Health, Baylor University, Waco, TX, USA
- Corresponding Author:
- Jeffrey S. Martin
Department of Basic Medical Sciences
DeBusk College of Osteopathic Medicine
Lincoln Memorial University
Knoxville TN, USA
E mail: jeffrey.martin@lmunet.edu
Received: 10-Jul-2023, Manuscript No. AAJPTSM-23-105475; Editor assigned: 11-Jul-2023, PreQC No. AAJPTSM-23-105475;(PQ); Reviewed: 18-Jul-2023, QC No AAJPTSM-23-105475; Published: 24-Jul-2023, DOI:10.35841/aajptsm-7.4.151
Citation: Martin JS, Morales GK, Bui JT, et al. Acute effects of a novel, calf-only external pneumatic compression device on measures of lower-limb blood flow, blood volume and tissue oxygenation. J Phys Ther Sports Med. 2023;7(4):151
Abstract
Background: We sought to determine the effects of a novel, calf-only sequential dynamic External Pneumatic Compression (EPC) device on lower limb blood flow and tissue oxygenation.
Methods: Thirty (N=30; EPC=20, sham=10) participants completed this randomized, shamcontrolled, single-visit study. Popliteal artery blood flow characteristics were measured prior to (PRE; 30-s duration), during (TRT; 210-s duration) and following (PST: 300-s duration) a 15-min EPC (target inflation pressure of ‘5’) or sham treatment. Additionally, Total Hemoglobin (THb) and Skeletal Muscle Oxygenation (SmO2) were measured continuously from the start of the study until 15-min following treatment.
Results: A significant group*time interaction was observed for antegrade and retrograde popliteal artery blood flow, max blood flow velocity and near infrared spectroscopy derived measures of calf THb and SmO2 (p<0.05 for all). Increases from PRE in antegrade (~28%) and retrograde popliteal artery blood flow (~95%) were significantly greater during EPC treatment, but not following treatment, compared to sham. Calf THb (~2%) and SmO2 (~10%) increases were significantly greater with EPC treatment compared to sham following treatment, but not during treatment. Finally, max blood flow velocity was significantly increased to a greater degree than sham both during (~105%) and following (~77%) EPC treatment.
Conclusion: A novel, calf-only sequential dynamic EPC device increases measures of antegrade and retrograde blood flow during treatment as well as blood volume and tissue oxygenation markers following treatment which may provide similar benefit to those observed previously with full-leg EPC alternatives.
Keywords
Vascular, Recovery, Sports, Muscle, Massage, Intermittent pneumatic compression, Health, Circulation.
Introduction
External Pneumatic Compression (EPC) devices have been widely utilized as a modality for decreasing post-exercise recovery time [1-5] and improving vascular biology in the lower extremities [6-9]. Indeed, the use of full leg dynamic, sequential EPC devices have been shown to decrease immediate post-workout blood lactate levels [3, 10], mitigate delayed onset muscle soreness [1, 11], increase flexibility [12] and reduce markers of proteolysis [13] following exercise. Moreover, full leg dynamic, sequential EPC has been shown to acutely improve flow mediated dilation, a surrogate of peripheral conduit function and reactivity [14]. Blood flow and muscle oxygenation are two variables that are often used as metrics to evaluate the efficacy of these devices, particularly with regards to claims of improving post-exercise recovery. In terms of human performance, increases in both blood flow [15, 16] and muscle oxygenation [17] have been implicated as significant factors in the recovery process, both of which are improved with full leg dynamic EPC [18, 19].
Recent technological advances have allowed for the development of more mobile and less cumbersome dynamic EPC devices. For example, calf only sequential pneumatic compression devices are now commercially available purporting similar benefits to those observed with the full leg EPC devices. The more mobile and portable nature of these devices is a benefit, but there is little evidence supporting claims that outcomes related to exercise recovery and limb blood flow are impacted to the same or a similar degree as observed with the full leg devices. Indeed, to our knowledge, the only study to-date regarding human performance, recovery and/or limb blood flow with dynamic, sequential calf only EPC devices demonstrated improvement in some countermovement jump testing performance characteristics following a damage protocol, but no significant differences in limb blood flow or muscle oxygenation at 15- and 30-min following treatment were observed with the EPC device [20]. Notably, blood flow and oxygenation were not measured during or immediately following treatment in this study, and the hemodynamics were confounded by the acute treatment protocol occurring immediately following an intense muscle damage protocol (100 drop jumps). Moreover, tissue oxygenation was measured upstream at the vastsus lateralis as opposed to where the pneumatic compression was actually applied (calf). Given the relative lack of literature regarding the effect of these devices on limb blood flow and muscle oxygenation, we sought to determine the isolated effects of calf only, dynamic, sequential pneumatic compression on lower limb blood flow and muscle tissue oxygenation. We hypothesized that blood flow markers measured via ultrasound at the popliteal artery and near infrared spectroscopy (NIRS) total hemoglobin (THb) measures of the gastrocnemius would be increased during and immediately following treatment. Similarly, we hypothesized that muscle oxygenation (SmO2) would be improved at the same time points.
Methods
Participants
Prior to initiating this study, the protocol was reviewed and approved by the Lincoln Memorial University Institutional Review Board (IRB #1154) and was in compliance with the ethical standards of the Helsinki Declaration. Thirty (N=30; females=17, males=13) apparently healthy volunteers were recruited from the local community through advertisement and word-of-mouth to participate in this single-blind, randomized study. Subjects were eligible to participate if they were between the ages of 18-64 years, not taking blood pressure medication and without history of bone, ligament or significant soft tissue injury to the lower limb(s) in the last 6 months. After providing their informed consent, all participants completed a medical history form to confirm eligibility and were scheduled to report for a single visit. For the experimental protocol visit, all participants were instructed to abstain from alcohol and caffeine for 24 and 12 hours prior, respectively.
Protocol
After confirmation of eligibility to participate in the study, participants were assigned a random participant ID number and randomized in a 2:1 manner to the EPC treatment or sham treatment group. Unequal randomization was utilized to improve the precision of the treatment effect. This design requires more subjects to achieve the same power as would be necessary with a 1:1 randomization, and therefore does not bias the study. After group assignment, participants had their height and weight (stadiometer) and left calf circumference (flexible measuring tape at largest circumference) measured and recorded. Thereafter, participants were fitted with a Near-Infrared Spectroscopy (NIRS) device (Moxy Monitor, Hutchinson, MN, USA) at the largest section of their left calf to continuously monitor muscle oxygenation (SmO2) and Total Hemoglobin (THb). Thereafter, the EPC device was placed over the left lower-limb between the ankle and knee and fitted using the inherent Velcro design. Following placement of the EPC calf sleeve, participants were asked to lay prone in a temperature control room for 5-min of rest. Thereafter, SmO2 and THb data collection began for another 5-min of rest (PRE timepoint) and continued throughout the end of the study. Lower limb blood flow was also assessed during the second 5-min rest period (PRE) via ultrasonography at the popliteal artery for 30-sec. Following the second 5-min rest period, the EPC device was turned on and EPC or sham treatment initiated. Approximately 5-min into the treatment period, blood flow in the popliteal artery was assessed again via ultrasound for 210 seconds (TRT period). The duration of this measure was to ensure an entire compression cycle was captured. Following the EPC/Sham treatment, blood flow in the popliteal artery was assessed for the final time for 5-min (PST period). SmO2 and THb data collection continued for 15- min following the treatment at which time the experimental protocol was concluded.
EPC and sham treatments
Participants randomized to the EPC treatment group received a single 15-min treatment to the left lower-limb with a mobile, dynamic and sequential pneumatic compression device (Normatec Go, Hyperice, Irvine, CA, USA) at a setting of “Level 5” for the target inflation pressure. This device is similar to the manufacturer’s original product (Normatec Legs) but instead of wrapping the entire lower limb, the device is wrapped around the leg only between the ankle and knee and without attachment to an air compressor. The lower-limb wrap includes 3 zones which inflate dynamically (i.e., peristaltic compression) and sequentially (distal to proximal); distal zone only, distal and medial zone, and all zones. Compression in each phase occurs for ~1-min followed by ~15s of deflation/ no compression resulting in a total cycle time of ~195 sec. 5 entire cycles are completed for a 15-min treatment as a cycle will complete itself when the timer reaches zero.
Participants randomized to the sham treatment group were fitted with the EPC device in the same fashion that the EPC treatment group was. However, the device was turned on but compression was not initiated. Participants were blinded to group assignment, though if they are knowledgeable about EPC products, were potentially aware of being in the sham group. Regardless, the sham treatment was employed, in part, to control for the simple effect of leg warming due to wearing an opaque device with power that could potentially increase skin/limb blood flow [14].
Popliteal artery blood flow
High resolution ultrasound (NextGen Logiq e R7; GE Healthcare, Chicago, IL USA) with a 4 to 13 MHz multifrequency linear phase array transducer was used to determine blood flow through the left popliteal artery at rest, during treatment and immediately following treatment. In brief, the artery was imaged longitudinally with the transducer placed 3-8 cm below the popliteal fossa and held manually in the same position for the duration of the measurements. Simultaneous measurement of artery diameter and blood velocity was performed using duplex mode imaging (B-mode and Doppler) and video was captured through a digital interface at 30 frames/second with real time analysis (FMD Studio v3, QUIPU, Pisa, Italy). Resting measurements were captured for 30-sec, treatment measures for 210-sec, and after the treatment concluded measurements were made for another 5-min. Vessel diameters were determined via automatic edge detection software (FMD Studio) measuring the distance between the near and far wall of the intima. Blood velocity was determined via selection of a region of interest around the Doppler waveform and a trace of the velocity-time integral was used to calculate positive and negative velocity for each cardiac cycle. Blood flow was calculated as [Π * (diameter/2)2 * time average mean velocity * 60].
Statistical analysis
All statistical analyses were performed using SPSS v22.0 (IBM Corp., Armonk, NY, USA). Prior to statistical analysis normality was confirmed using Shapiro-Wilk tests. For ANOVA, if sphericity was violated a correction factor was applied (i.e., Huynh-Feldt) to hypothesis testing. Participant characteristics between groups were analyzed using Satterthwaite t-tests for unequal variance. For all physiological dependent variables, two-way repeated measures ANOVAs were performed with time (PRE, TRT and PST) and group (EPC vs. sham) as independent variables. When a significant time*group interaction was observed, post-hoc comparisons were made using Bonferroni corrected (α/[number of comparisons-1]) Satterthwaite t-tests comparing changes scores from PRE at the TRT and PST time points.
Results
Participant characteristics
Participant characteristics are reported in (Table 1). There was no significant difference in sex, BMI (Range: EPC, 18.0-37.8 kg/m2; sham, 20.2-30.1 kg/m2), height (EPC, 1.55- 1.93 m; sham, 1.55-1.78 m), weight (EPC, 47.7-129.3 kg; sham, 48.5-95.3 kg) or calf circumference (EPC, 31.0-43.5 cm; sham, 32.0-41.0 cm) between EPC and sham treatment groups. There was a significant difference in age with the EPC treatment group being, on average, ~8.4 years older (Range:EPC, 23-52 years; sham, 22-27 years). However, age was not found to be a significant covariate in the statistical model, nor was sex, BMI or calf circumference.
Effects of calf only EPC on popliteal artery blood flow
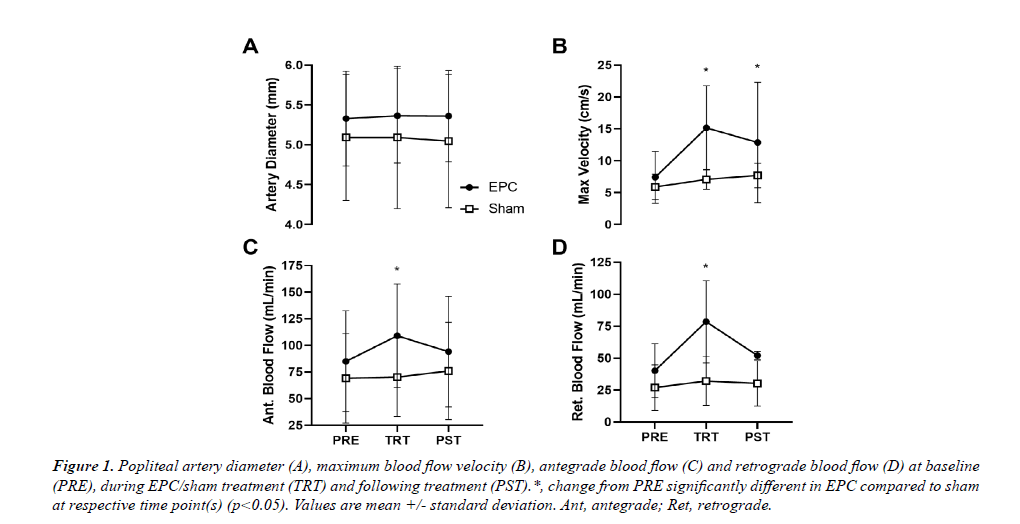
For popliteal artery diameter, no significant main effect of time, group or their interaction was observed (p>0.05; Figure 1A). For mean blood flow, no main effect of group or time*group interaction was observed (p>0.05), but there was a significant main effect of time (p=0.045). However, post-hoc analysis revealed no significant between timepoints differences in mean popliteal artery blood flow across groups (p>0.05). For popliteal artery maximum blood flow velocity (Figure 1B), antegrade blood flow (Figure 1C) and retrograde blood flow (Figure 1D), a significant time*group interaction was observed (p=0.011, p=0.038, and p<0.001, respectively). For maximum blood flow velocity, change from PRE during (TRT: p<0.001) and following treatment (PST: p=0.024) was significantly greater in the EPC group compared to sham (+104.8 ± 61.8% and +77.4 ± 89.3% for EPC and +19.9 ± 31.4% and +30.8 ± 25.3% for sham). Change from PRE in popliteal artery antegrade blood flow was significantly greater in the EPC group compared to sham during TRT (p=0.024; +28.3 ± 33.3% and +1.65 ± 31.1% for EPC and sham, respectively), but not over the 5-minute measurement period PST-treatment (p=0.922; +9.21 ± 33.1% and +10.0 ± 36.8%). Similarly, change from PRE in popliteal artery retrograde blood flow was significantly greater in the EPC group compared to sham during TRT (p<0.001; +95.1 ± 65.9% and +18.9 ± 35.3% for EPC and sham, respectively), but not over the course of the PST-treatment measurement period (p=0.922; +27.5 ± 41.1% and +11.9 ± 44.4%).
Figure 1: Popliteal artery diameter (A), maximum blood flow velocity (B), antegrade blood flow (C) and retrograde blood flow (D) at baseline (PRE), during EPC/sham treatment (TRT) and following treatment (PST).*, change from PRE significantly different in EPC compared to sham at respective time point(s) (p<0.05). Values are mean +/- standard deviation. Ant, antegrade; Ret, retrograde.
Effects of calf only EPC on SmO2 and THb
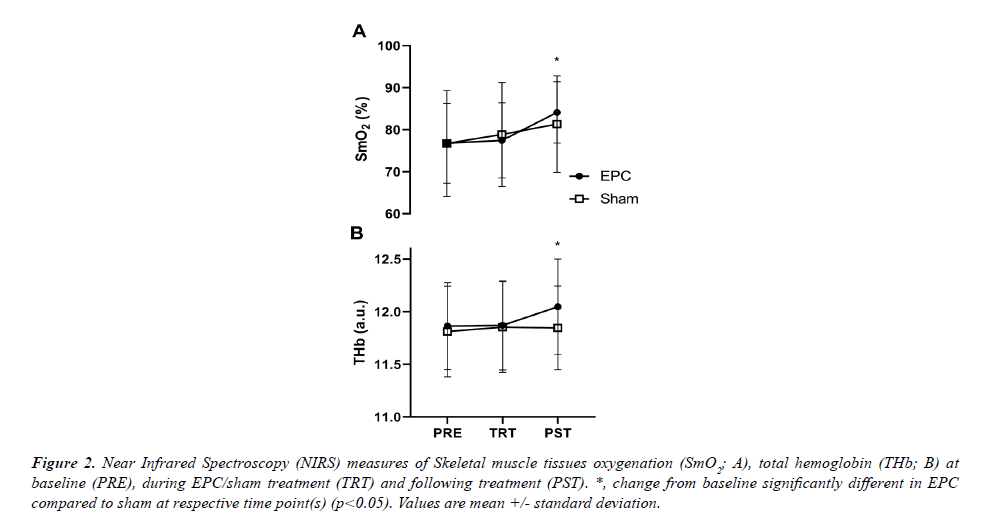
A significant group*time interaction was observed for both SmO2 (p=0.043) and THb (p<0.001) derived from NIRS measurements (Figure 2A). Post-hoc analysis revealed that SmO2 increases from PRE were significantly greater in the EPC group compared to sham for PST treatment (Fig. 2A; p=0.019; +9.5 ± 7.4% and +6.1 ± 2.6% for EPC and sham, respectively), but not during TRT (p=0.296; +0.8 ± 5.5% and +2.8 ± 2.6%). Similarly, THb increases from PRE were significantly greater in EPC compared to sham at the during the PST-treatment measurement (Figure 2B; p=0.006; +1.6 ± 0.8% and +0.4 ± 0.5% for EPC and sham, respectively), but not during TRT (p=0.183; +0.1 ± 0.5% and +0.4 ± 0.5%).
Figure 2: Near Infrared Spectroscopy (NIRS) measures of Skeletal muscle tissues oxygenation (SmO2; A), total hemoglobin (THb; B) at baseline (PRE), during EPC/sham treatment (TRT) and following treatment (PST). *, change from baseline significantly different in EPC compared to sham at respective time point(s) (p<0.05). Values are mean +/- standard deviation.
Discussion
The primary findings of the present study are that, compared to sham, a novel, calf-only, sequential dynamic EPC device 1) increases antegrade and retrograde popliteal artery blood flow during TRT, 2) increases calf SmO2 and THb in the 15-min following treatment (PST) and 3) increases peak blood flow velocity during and after TRT.
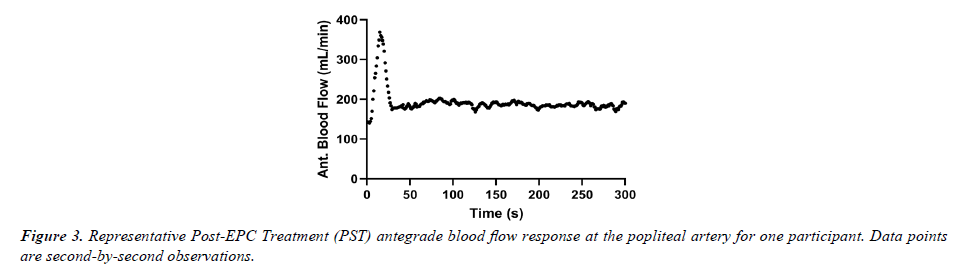
We hypothesized that blood flow markers measured via ultrasound at the popliteal artery would be increased during and immediately following TRT but found a significant increase only during TRT compared to sham. The findings during TRT were similar to a previous study with full-leg, sequential dynamic EPC [19]. Moreover, it was notable during data analysis that there was a robust increase in antegrade (and mean) popliteal artery blood flow immediately following cessation of the treatment, but the response was short-lived (~30 sec). Figure 3 provides an example of one participant’s data for second-by-second blood flow in the popliteal artery during the PST time period. Given the relatively small portion of the PST period, the transient nature of the reactive hyperemia observed was likely “washed-out” over the course of the entire PST period. This observation is supported by the nearly two-fold (77%) increase in maximum blood flow velocity observed during the post- EPC TRT period.
THb, a marker of blood volume from NIRS measurements at the compressed calf, and SmO2, a marker of muscle tissue oxygenation, were increased with EPC compared to sham in the PST period, but not during TRT. The the lack of significant change during the TRT period aligns with the mean blood flow changes observed with popliteal artery blood flow (net of antegrade and retrograde blood flow) and the mobilization of fluid from the tissue space with compression. Moreover, considering the duty cycle of the device, only ~8% of an entire cycle was dedicated to cuff release/decompression. Thus, the strong majority of the entire cycle that was measured was associated with tissue compression which provides resistance to blood flow [21] while also affecting hemodynamics [19] without potentially allowing a complete reactive hyperemic response before another compression cycle begins [22]. This is further supported by the duration of hyperemia observed in the PST period (Figure 3). For THb and SmO2, in the post-treatment period, the data was in contrast with the popliteal artery blood flow data. However, the PST-EPC treatment increase in THb and SmO2 was consistent with previous findings with full-leg dynamic EPC [18]. Potential reasons for the discrepancy with the ultrasound derived measures of popliteal artery blood flow include 1) the potential for cutaneous blood flow contributing to the NIRS-derived observations and magnitude thereof [23] and 2) the location of the measurement (calf tissue vs. upstream popliteal artery). While our findings are in contrast to those observed by Blumkaitis et al [20], their comparisons to a control condition were confounded by an exercise damage protocol immediately prior to treatment with a calf-only EPC device as well as the fact that the NIRS measures were performed at the mid-thigh. Notably, heating the tissue could have a significant impact on NIRS measurements [24, 25], thus the application of a sham was important to reduce the effect of a thermal load due to wearing an opaque material alone. Regardless, the compressive nature of EPC may have further increased heat load during a passive state and thus contributed to the observed THb response [26].
Limitations
Among the limitations of the current study is that only a single target inflation pressure was used for the EPC treatments. Previous studies have shown that there can be differential responses in hemodynamic changes with different target inflation pressures and compression durations [19, 27]. However, the target inflation pressure employed herein was a “moderate” intensity to illustrate an average response to the EPC treatment. Regardless, future studies should characterize the potential impact of target inflation pressures on the outcomes reported. Another potential limitation of the present study was the duration of the measurement periods. While the PRE and TRT periods were appropriate, the post-treatment measurement period would benefit from future studies describing the duration of the effect on THb and SmO2 as 15-min may not have been sufficient to document a return to PRE or similar change from PRE as the sham treatment. There was a significant age difference between the two treatment groups, but age was not found to be a significant covariate in the statistical model. However, the relatively small sample size of the present investigation may have limited the ability to detect small differences due to age, sex, calf circumference, etc. Moreover, the relatively heterogenous population included in this study improves applicability. Finally, the protocol employed herein was in the context of being in a rested, prone condition. The potential effects of exercise in the hours and/or days preceding the EPC treatment, as well as the posture during treatment, may impact the hemodynamic changes observed.
Conclusion
A single, 15-min, moderate target inflation pressure treatment with a novel, calf-only, dynamic sequential EPC device significantly increases antegrade and retrograde blood flow. It also acutely increases markers of blood volume and compressed tissue oxygenation in the 15-min following treatment. While future studies should be conducted regarding the minimum and/or maximum target inflation pressure required to significantly increase blood flow, blood volume and oxygenation characteristics, the results herein suggest that calf-only, dynamic sequential EPC may be a viable alternative to the comparable full-leg devices. The increased portability, mobility and convenience of these devices may provide more options to trainers and health care professionals in the sports recovery and/or health domains.
Acknowledgments
The authors wish to thank the participants for their compliance and for devoting their time to this study.
Conflicts of Interest
J.S.M. is a paid consultant for Hyperice, though no funding or pay for performance of this study nor the writing of this manuscript was provided. All other authors have no conflicts of interest to disclose.
References
- Haun CT, Roberts MD, Romero MA, et al. Does external pneumatic compression treatment between bouts of overreaching resistance training sessions exert differential effects on molecular signaling and performance-related variables compared to passive recovery? An exploratory study. PLoS One. 2017;12(6):e0180429.
- Hoffman MD, Badowski N, Chin J, et al. A randomized controlled trial of massage and pneumatic compression for ultramarathon recovery. J Orthop Sports Phys Ther. 2016;46(5):320-6.
- Martin JS, Friedenreich ZD, Borges AR, et al. Acute effects of peristaltic pneumatic compression on repeated anaerobic exercise performance and blood lactate clearance. J Strength Cond Res. 2015;29(10):2900-6.
- Rahman M, Ahmad I, Hussain ME. Comparison of intermittent pneumatic compression and active recovery after sub-maximal aerobic exercise in collegiate soccer players: in relation with heart rate variability and heart rate recovery. Sport Sci Health. 2022;18(4):1349-58.
- Wisniowski P, Cieslinski M, Jarocka M, et al. The effect of pressotherapy on performance and recovery in the management of delayed onset muscle soreness: A systematic review and meta-analysis. J Clin Med. 2022;11(8):2077.
- Credeur DP, Vana LM, Kelley ET, et al. Effects of intermittent pneumatic compression on leg vascular function in people with spinal cord injury: a pilot study. J Spinal Cord Med. 2019;42(5):586-94.
- Martin JS, Borges AR, Beck DT. Peripheral conduit and resistance artery function are improved following a single, 1-h bout of peristaltic pulse external pneumatic compression. Eur J Appl Physiol. 2015;115:2019-29.
- Sheldon RD, Roseguini BT, Laughlin MH, et al. New insights into the physiologic basis for intermittent pneumatic limb compression as a therapeutic strategy for peripheral artery disease. J Vasc Surg. 2013;58(6):1688-96.
- Kephart WC, Mobley CB, Fox CD, et al A single bout of whole-leg, peristaltic pulse external pneumatic compression upregulates PGC-1a mRNA and endothelial nitric oxide sythase protein in human skeletal muscle tissue. Exp Physiol. 2015;100(7):852-64.
- Hanson E, Stetter K, Li R, et al. An intermittent pneumatic compression device reduces blood lactate concentrations more effectively than passive recovery after Wingate testing. J Athl Enhanc. 2013;2(3):18-25.
- Sands WA, McNeal JR, Murray SR, et al. Dynamic compression enhances pressure-to-pain threshold in elite athlete recovery: exploratory study. J Strength Cond Res. 2015;29(5):1263-72.
- Sands WA, Murray MB, Murray SR, et al. Peristaltic pulse dynamic compression of the lower extremity enhances flexibility. J Strength Cond Res. 2014;28(4):1058-64.
- Haun CT, Roberts MD, Romero MA, et al. Concomitant external pneumatic compression treatment with consecutive days of high intensity interval training reduces markers of proteolysis. Eur J Appl Physiol. 2017;117:2587-600.
- Martin JS, Martin AM, Mumford PW, et al. Unilateral application of an external pneumatic compression therapy improves skin blood flow and vascular reactivity bilaterally. PeerJ. 2018;6:e4878.
- Borne R, Hausswirth C, Bieuzen F. Relationship between blood flow and performance recovery: a randomized, placebo-controlled study. Int J Sports Physiol Perform. 2017;12(2):152-60.
- O’Riordan SF, Bishop DJ, Halson SL, Broatch JR. Compression-induced improvements in post-exercise recovery are associated with enhanced blood flow, and are not due to the placebo effect. Sci Rep. 2022;12(1):16762.
- Tan Q, Wang Y, Chen TL, et al. Exercise-induced hemodynamic changes in muscle tissue: Implication of muscle fatigue. App Sci. 2020;10(10):3512.
- Brock KA, Eberman LE, Laird RH, et al. Sequential pulse compression’s effect on blood flow in the lower-extremity. J Sport Rehabil. 2020;29(1):7-11.
- Martin JS, Kephart WC, Haun CT, et al. Impact of external pneumatic compression target inflation pressure on transcriptome-wide RNA expression in skeletal muscle. Physiol Rep. 2016;4(22):e13029.
- Blumkaitis JC, Moon JM, Ratliff KM, et al. Effects of an external pneumatic compression device vs static compression garment on peripheral circulation and markers of sports performance and recovery. Eur J Appl Physiol. 2022;122(7):1709-22.
- Nielsen HV. Effects of externally applied compression on blood flow in the human dependent leg. Clin Physiol. 1983;3(6):131-40.
- Betik AC, Luckham VB, Hughson RL. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol. 2004;286(1):H442-8.
- Bartlett MF, Akins JD, Oneglia AP, et al. Impact of cutaneous blood flow on NIR-DCS measures of skeletal muscle blood flow index. J Appl Physiol. 2021;131(3):914-26.
- Davis SL, Fadel PJ, Cui J, et al. Skin blood flow influences near-infrared spectroscopy-derived measurements of tissue oxygenation during heat stress. J Appl Physiol. 2006;100(1):221-4.
- Tew GA, Ruddock AD, Saxton JM. Skin blood flow differentially affects near-infrared spectroscopy-derived measures of muscle oxygen saturation and blood volume at rest and during dynamic leg exercise. Eur J Appl Physiol. 2010;110:1083-9.
- Pearson J, Low DA, Stöhr E, et al. Hemodynamic responses to heat stress in the resting and exercising human leg: insight into the effect of temperature on skeletal muscle blood flows. Am J Physiol Regul Integr Comp Physiol. 2011;300(3):R663-73.
- Kakkos SK, Nicolaides AN, Griffin M, et al. Comparison of two intermittent pneumatic compression systems: a hemodynamic study. Int Angiol. 2005;24(4):330.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref