- Biomedical Research (2011) Volume 22, Issue 3
Activity induced by a danazol derivative on perfusion pressure and coro-nary resistance in isolated rat heart
Figueroa-Valverde Lauro1*, Díaz-Cedillo Francisco2, Lopez-Ramos Ma1, Garcia-Cervera Elodia11Laboratorio de Investigación de la Facultad de Ciencias Químico-Biológicas, Universidad Autónoma de Campeche, Av. Agustín Melgar, Col Buenavista C.P.24039 Campeche Cam., México. lauro_1999@yahoo.com
2Laboratorio de Química Orgánica de la Esc. Nal. de Ciencias Biológicas del Instituto Politécnico Nacional. Prol. Carpio y Plan de Ayala s/n Col. Santo Tomas, México, D.F. C.P. 11340.stybium@yahoo.com
- *Corresponding Author:
- Figueroa-Valverde Lauro
Laboratorio de Investigación de la Facultad de Ciencias Químico-Biológicas
Universidad Autónoma de Campeche
Av. Agustín Melgar
Col Buenavista C.P.24039 Campeche Cam.,México
Accepted February 15 2011
Abstract
Experimental studies suggest that danazol can be associated with changes in blood pressure. Nevertheless, there is scarce information about the effects of danazol and its derivatives at cardiovascular level. To clarify on this phenomenon, we evaluated the effects of danazol de-rivative on perfusion pressure in isolated rat heart using Langendorff flow model. Our re-sults demonstrated that pregnenolone-derivative (10-9 mM) significantly increase the perfu-sion pressure (p = 0.05) and vascular resistance (p = 0.05) in isolated heart. The activity in-duced by danazol derivative on perfusion pressure (10-9 to 10-4 mM) was blocked in presence of nifedipine (10-6 mM).These data suggest that activity induced by danazol derivative on perfusion pressure and vascular resistance is dependent upon its chemical structure. This phenomenon involves the L-type calcium channel activation through a non-genomic molecu-lar mechanism
Keywords
Danazol, derivative, Langendorff, perfusion pressure
Introduction
High blood pressure contributes substantially to cardio-vascular disease incidence and premature mortality [1,2]. Studies using the technique of ambulatory blood pressure monitoring have shown that blood pressure is higher in men than in women of similar ages [3,4]. Experimental and clinical studies [5-7] has demonstrated that androgens can be associated with hypertension development. In particular, there are some reports which indicate that danazol is associated with hypertension [8]. For example the studies reported by Wu and coworkers [9] who sug-gest that there is a relationship between the danazol and hypertension. These data are supported by the studies of Bretza and coworkers [10]. which indicate that admini-stration of danazol increase the blood pressure, this phe-nomenon was associated with changes in the sodium levels. Nevertheless, it is important to mention that other clinical data show that activity induced by danazol could be an independent factor of hypertension associated to arterial thrombosis [11].
Recently, a study [12] showed that a danazol derivative (hemisuccinate of danazol) increases the perfusion pres-sure and resistance vascular in isolated rat heart via inter-action of androgen-receptor.
Apart from the above experiments, which also do not show clearly the cellular site and actual molecular mecha-nisms of danazol and its derivatives, data information are needed for characterizing the activity induced by this steroid and its derivatives at cardiovascular level. To provide this information, the present study was designed to investigate the effects of danazol and the danazol de-rivative (PDE) on perfusion pressure and coronary resis-tance in isolated rat hearts using Langendonff model [13]. Additionally, the molecular mechanism involved in the activity induced by PDE on perfusion pressure was evalu-ated using several substances such as flutamide (androgen receptor antagonist) [14], prazosin (α1 adrenoreceptor antagonist) [15], metoprolol (selective β1 receptor blocker) [16] and nifedipine (antagonist calcium channel type L) [17] as pharmacological tools.
Material and Methods
General methods
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal Care and Use Committee of Universidad Autonoma de Campeche (UAC) and were in accordance with the Guide for the Care and Use of Laboratory Animals [18]. . Male rats (Wistar; weighing 200-250 g; n = 63) were obtained from UAC.
Reagents
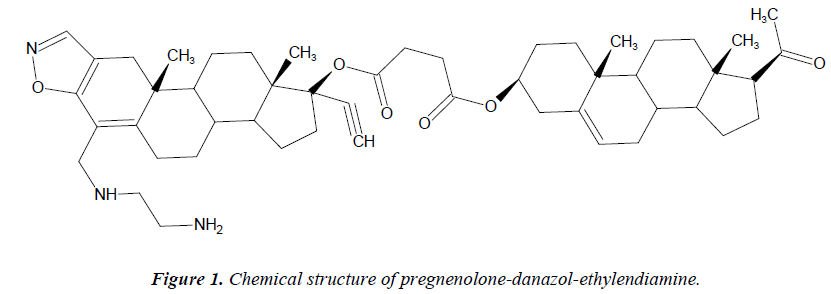
Pregnenolone-danazol-ethylendiamine conjugate (suc-cinic acid 6-[2-amino-ethylamino)-methyl]-2-ethynyl-10a,12a-di-methyl-2,3,3-3b-,5,10,10a,10b,11,12,12a, dod-ecahydro-1H-7-oxa-8-aza-dicyclopenta[a,h] phenanthren-1-yl-ester-17-acetyl,10,13-dimethyl-2,3,4,7,8,9, 10,11, 12, 13,14,15,16,17-tetradecahydro-1-H-cyclopenta [a] phe-nanthren- 3-yl ester) showed in Figure 1 was prepared according to a previously reported method by Figueroa and coworkers [19]. Other reagents were obtained from Sigma-Aldrich Chemical Co. All drugs were dissolved in methanol and different dilutions were obtained using Krebs-Henseleit solution (0.01%, v/v).
Langendorff method
Briefly, the male rat (200 - 250 g) was anesthetized by injecting them with pentobarbital at a dose of 50 mg/Kg body weight. The chest was opened and a loose ligature passed through the ascending aorta. The heart was then rapidly removed and immersed in ice cold physiologic saline solution. The heart was trimmed of non-cardiac tissue and retrograde perfused via a noncirculating perfu-sion system at a constant flow rate. The perfusion me-dium was the Krebs-Henseleit solution (pH 7.4, 37°C) composed of (mM); 117.8 NaCl; 6 KCl; 1.75 CaCl2; 1.2 NaH2PO4; 1.2 MgSO4; 24.2 NaHCO3; 5 glucose and 5 sodium pyruvate. The solution was actively bubbled with a mixture of O2/CO2 (95:5).
The coronary flow was adjusted with a variable-speed peristaltic pump. An initial perfusion rate of 15 ml/min for 5 min was followed by a 25 min equilibration period at a perfusion rate of 10 ml/min. All experimental meas-urements were done within the equilibration period.
Perfusion pressure
Evaluations of perfusion pressure changes induced by drugs in this study were assessed using a pressure trans-ducer connected to the chamber where the hearts were mounted and the results entered into a computerized data capture system (Biopac).
Biological evaluation
Effect induced by pregnenolone, danazol and PDE on perfusion pressure
Time course changes in perfusion pressure of pregne-nolone, danazol and PDE at a concentration of 10-9 mM were determined. The effects were obtained in isolated hearts perfused at a constant-flow rate of 10 ml/min. It is important to mention that conditions used were manly the previous reports [20].
Evaluation of effects exerted by pregnenolone, danazol and PDE on coronary resistance
The coronary resistance in absence (control) or presence of pregnenolone, danazol and PDE at a concentration of 10-9 mM was evaluated. The effects were obtained in isolated hearts perfused at a constant flow rate of 10 ml/min. The coronary resistance was determined by the relationship between coronary flow and perfusion pres-sure (mm Hg/ml/min) [21,22].
Effects induced by PDE on perfusion pressure through androgen receptors
Intracoronary boluses (50 μl) of PDE [10-9 to 10-4 mM] were administered and the corresponding effect on the perfusion pressure was determined. The dose-response curve (control) was repeated in the presence of flutamide at a concentration of 10-6 mM (duration of preincubation with flutamide was by a 10 min equilibration period). The dose of adrenergic antagonist was using previous reports [23]
Effect exerted by PDE on perfusion pressure in the presence of α1 adrenergic blocker
The boluses (50 μl) of PDE [10-9 to 10-4 mM] were ad-ministered and the corresponding effect on the perfusion pressure was evaluated. It is important to mention that the bolus injection administered was done in the point of cannulation. The dose-response curve (control) was re-peated in the presence of prazosin at a concentration of 10-6 mM (duration of preincubation with prazosin was by a 10 min equilibration period). The dose of α1 adrenergic antagonist was using the reports by Figueroa [23] Drew [24].
Effects induced by PDE on perfusion pressure in the presence of β1 adrenergic blocker
The boluses (50 μl) of PDE [10-9 to 10-4 mM] were ad-ministered and the corresponding effect on the perfusion pressure was evaluated. The dose-response curve (con-trol) was repeated in the presence of metoprolol at con-centration of 10-6 mM (duration of preincubation with metoprolol was by a 10 min equilibration period). The dose of β1 adrenergic antagonist was using previous re-ports [21,22,25].
Activities exerted by PDE on perfusion pressure in the presence of calcium channel blocker
The boluses (50 μl) of PDE [10-9 to 10-4 mM] were ad-ministered and the corresponding effect on the perfusion pressure was evaluated. The dose-response curve (con-trol) was repeated in the presence of nifedipine at a con-centration of 10-6 mM (duration of preincubation with nifedipine was by a 10 min equilibration period). The dose of calcium antagonist was using previous reports [24].
Statistical analysis
The obtained values are expressed as mean ± SE (stan-dard error), using each heart as its own control. The com-paration between means was made with a paired Stu- dent’s t test. In the case multiple comparation was used an analysis of variance (ANOVA) using the Bonferroni cor-rection factor26. The differences were considered signifi-cant when p was equal or smaller than 0.05.
Results
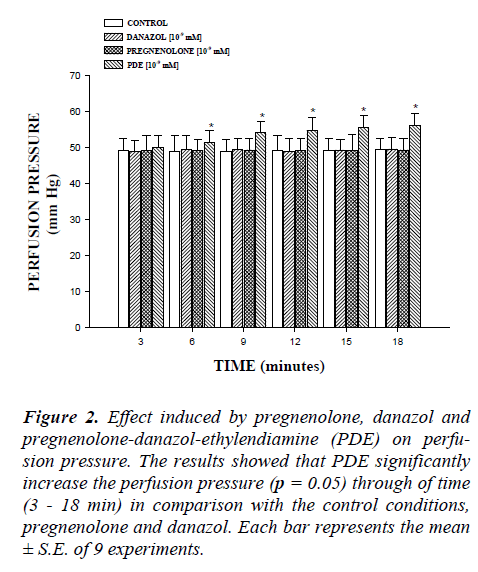
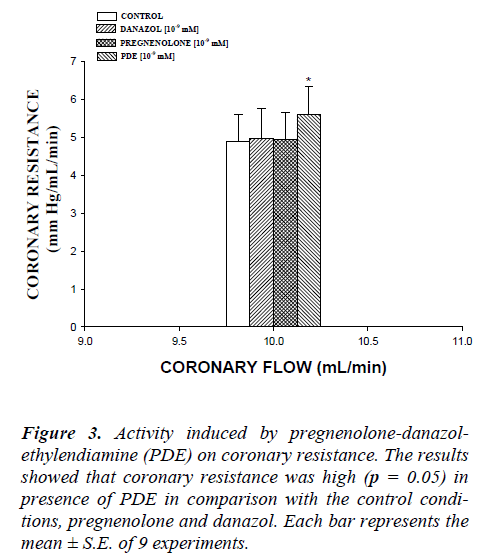
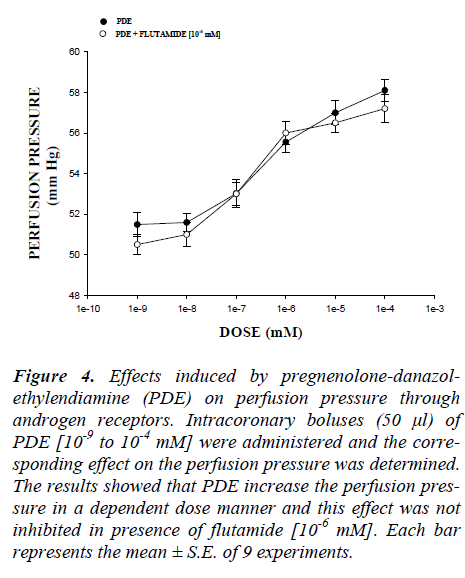
In this study, the activity induced by danazol, pregneno-lone and pregnenolone-danazol-ethylendiamine conjugate (PDE) on perfusion pressure and coronary resistance in isolated rat heart was evaluated. The results obtained from changes in perfusion pressure as a consequence of in-creases in the time (3-18 min) in absence (control) or in presence of pregnenolone, danazol and PDE (Figure 2), showed that PDE [10-9 mM] significantly increase the perfusion pressure (p = 0.05) in comparison with the con-trol conditions, pregnenolone and danazol at the same dose. Additionally, another result showed that coronary resistance, calculated as the ratio of perfusion pressure at coronary flow assayed (10 ml/min) was higher in the presence of PDE than in control conditions, pregnenolone and danazol (p = 0.05) at a concentration of 10-9 mmol (Figure 3). Another result (Figure 4) showed that PDE increase the perfusion pressure in a dose dependent man-ner [10-9 to 10-4 mM] and this effect was not inhibited in presence of flutamide [10-6 mM].
Figure 2: Effect induced by pregnenolone, danazol and pregnenolone-danazol-ethylendiamine (PDE) on perfu-sion pressure. The results showed that PDE significantly increase the perfusion pressure (p = 0.05) through of time (3 - 18 min) in comparison with the control conditions, pregnenolone and danazol. Each bar represents the mean ± S.E. of 9 experiments.
Figure 3: Activity induced by pregnenolone-danazol-ethylendiamine (PDE) on coronary resistance. The results showed that coronary resistance was high (p = 0.05) in presence of PDE in comparison with the control condi-tions, pregnenolone and danazol. Each bar represents the mean ± S.E. of 9 experiments.
Figure 4: Effects induced by pregnenolone-danazol-ethylendiamine (PDE) on perfusion pressure through androgen receptors. Intracoronary boluses (50 μl) of PDE [10-9 to 10-4 mM] were administered and the corre-sponding effect on the perfusion pressure was determined. The results showed that PDE increase the perfusion pres-sure in a dependent dose manner and this effect was not inhibited in presence of flutamide [10-6 mM]. Each bar represents the mean ± S.E. of 9 experiments.
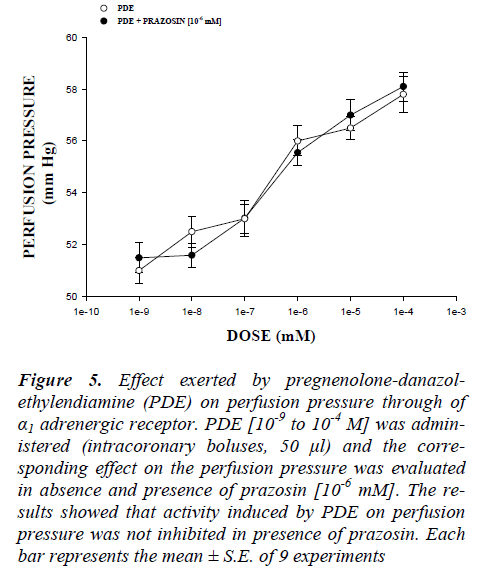
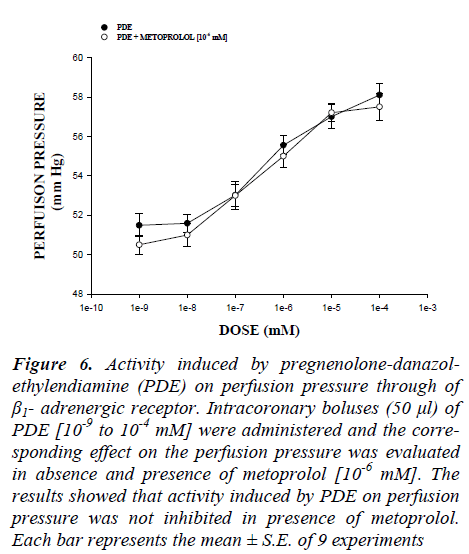
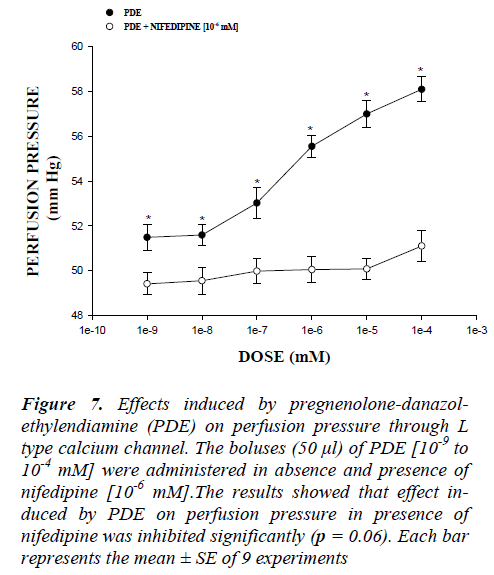
On the other hand, other experiments showed that PDE increase the perfusion pressure in a dose dependent man-ner [10-9 to 10-4 mM] and this effect was not inhibited in presence of prazosin (Figure 5) and metoprolol (Figure 6) at a concentration of 10-6 mM. Alternative experimental indicate that the activity exerted by PDE [10-9 to 10-4 mM] on perfusion pressure (Figure 7) in presence of nifedipine at a concentration of 10-6 mM was significantly inhibited (p = 0.06).
Figure 5: Effect exerted by pregnenolone-danazol-ethylendiamine (PDE) on perfusion pressure through of α1 adrenergic receptor. PDE [10-9 to 10-4 M] was admin-istered (intracoronary boluses, 50 μl) and the corre-sponding effect on the perfusion pressure was evaluated in absence and presence of prazosin [10-6 mM]. The re-sults showed that activity induced by PDE on perfusion pressure was not inhibited in presence of prazosin. Each bar represents the mean ± S.E. of 9 experiments
Figure 6: Activity induced by pregnenolone-danazol-ethylendiamine (PDE) on perfusion pressure through of β1- adrenergic receptor. Intracoronary boluses (50 μl) of PDE [10-9 to 10-4 mM] were administered and the corre-sponding effect on the perfusion pressure was evaluated in absence and presence of metoprolol [10-6 mM]. The results showed that activity induced by PDE on perfusion pressure was not inhibited in presence of metoprolol. Each bar represents the mean ± S.E. of 9 experiments
Figure 7: Effects induced by pregnenolone-danazol-ethylendiamine (PDE) on perfusion pressure through L type calcium channel. The boluses (50 μl) of PDE [10-9 to 10-4 mM] were administered in absence and presence of nifedipine [10-6 mM].The results showed that effect in-duced by PDE on perfusion pressure in presence of nifedipine was inhibited significantly (p = 0.06). Each bar represents the mean ± SE of 9 experiments
Discussion
In this study, was evaluated the effect of danazol derivative on blood vessel capacity and coronary resis-tance translated as changes in perfusion pressure in iso-lated rat heart (Langendorff model). The results show that danazol derivative significantly increased perfusion pres-sure over time (3-18 min) compared to the control condi-tions. It is important to mention that analyzing the possi-bility of that both danazol and pregnenolone fragments involved in the chemical structure of danazol derivative could be the responsible of the activity induced by this steroid derivative on the perfusion pressure, the effects exerted by pregnenolone and danazol on the perfusion pressure were evaluated to compare with the activity induced by danazol derivative. The results indicate that perfusion pressure was not affected in presence of both danazol and pregnenolone in comparison with the control conditions. Those experimental data indicate that the steroid derivative induce effects on perfusion pressure, which could consequently bring modifications in coro-nary resistance as it happens in another type of steroid derivatives [20-23]. To test this hypothesis, we evaluated the effects induced by danazol derivative on coronary resis-tance. We found that coronary resistance was increased by the danazol derivative in comparison with pregnenolone, danazol and control conditions. These data suggest that steroid derivative exerts effect on vascular tone. To characterize the molecular mechanism of this phenomenon we noted the reports of some investiga-tions [27,28] which indicate that some androgens induce its effect on blood pressure via activation of the androgen receptor [27]. For this reason, we used flutamide (androgen receptor blocker) to determine if the effects of danazol derivative on perfusion pressure were via the androgen receptor activation as in the case of other androgen de-rivatives [21-23]. Our results showed that the effects of dana-zol derivative were not inhibited by flutamide, suggesting that the molecular mechanism is not via the androgen-receptor.
On the other hand, analyzing data obtained in this study and the report on the molecular mechanism proposed by Kumai and coworkers [28] which suggests that some steroids can exert an indirect tonic effect on adrenal catecholamine synthesis and secretion. In Addition, of other studies which suggest that androgens stimulate the increased expression of adrenergic receptors (in some cellular lines), which has an important role in the development or maintenance of elevated blood pressure [29]. To evaluate this hypothesis in this study, the effect exerted by danazol derivative on perfusion pressure was evaluated in absence or presence of prazosin (α1 adrenoreceptor antagonist) and metoprolol (selective β1 receptor blocker). Our results showed that the effect induced by the danazol derivative was not inhibited in presence of these compounds. These data indicate that molecular mechanism involved in the effects of this danazol derivative on perfusion pressure is not through adrenergic activity.
Therefore, analyzing experimental data obtained in this study, we also considered validating the effect induced by some steroids on perfusion pressure via calcium-channels [30]. To evaluate this hypothesis in this study, the effect induced by danazol derivative on perfusion pres-sure was evaluated in absence or presence nifedipine (antagonist type L calcium-channel). The results showed that activity of steroid derivative in presence of nifedipine was blocked significantly. These data are similar to some reports [23] which showed that the pressor effect of some steroid derivatives is partially reversed by the subsequent administration of nifedipine. In conclusion, the results obtained suggest that activity induced by danazol deriva-tive on perfusion pressure and vascular resistance is de-pendent upon its chemical structure. This phenomenon involves activation of the L-type calcium channel via a non-genomic molecular mechanism
Acknowledgment
We are grateful to Enriqueta Valverde Anzurez, Glafira Valverde Anzurez, and Gloria Velazquez Zea for techni-cal assistance.
References
- Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arterioscler 1989; 9: 119-132.
- Mahoney L, Burns T, Stanford W. Coronary risk fac- tors measured in childhood and young adult life are as- sociated with coronary artery calcification in young adults. J Am Coll Cardiol 1996; 27: 277-284.
- Khoury S, Yarows S, O´brien T, et al. Ambulatory blood pressure monitoring in a nonacademic setting: ef- fects of age and sex. Am J Hypertens 1992; 5: 616-623.
- Wiinberg N, Hoegholm A, Christensen H, et al. 24-hAmbulatory blood pressure in 352 normal Danish sub- jects, related to age and gender. Am J Hypertens 1995; 8: 978-986.
- Barrett C, Khaw K. Endogenous sex hormones and cardiovascular disease in men. A prospective popula- tionbased study. Circulation 1988; 78: 539-545.
- Figueroa-Valverde L, Maldonado G, Díaz E, Ozaeta R. Evaluación de la actividad biológica de la testosterona sobre la presión de perfusión en un modelo de corazón aislado. Rev Inter Androl 2008; 6(4): 236-241.
- Svartberg J, Muhlen1 D, Schirmer H, et al. Association of endogenous testosterone with blood pressure and leftventricular mass in men. The Tromsø Study. Eur J En- docrinol 2004; 150: 65-71.
- Pears J, Sandercock P. Benign intracranial hypertension associated with danazol. Scott Med J 1990; 35:49.
- Wu Y, Chen S, Li T, et al. Intracranial hypertensionassociated with danazol withdrawal: a case report. Acta Neurol Taiwan 2007; 16: 173-176.
- Bretza J, Novey H, Vaziri N. Hypertension: a compli- cation of danazol therapy. Arch Intern Med 1980; 140: 1379-1380.
- Alvarado R, Liu J, Zwolak R. Danazol and limb-threa-tening arterial thrombosis: two case reports. J Vasc Surg 2001; 34: 1123-1126.
- Figueroa-Valverde L, Diaz-Ku E, Díaz-Cedillo F, et al.Effects of danazol and danazol hemisuccinate on perfu- sion pressure and vascular resistance. Acta Bioquím Clín Latinoam 2010; 44 (1): 37-45.
- Figueroa-Valverde L, Luna L, Castillo-Henkel C, et al.Synthesis and evaluation of the cardiovascular effects of two, membrane impermeant, macromolecular com- plexes of dextran-testosterone. Steroids 2002; 67: 611-619.
- Kontula K, Seppanen P, Duyne P, et al. Effect of a nonsteroidal antiandrogen, flutamide, on androgen re-ceptor dynamics and ornithine decarboxylase gene ex-pression in mouse kidney. Endocrinol 1985; 116: 226-233.
- Graham R, Oates H, Stoker L, et al. Alpha blocking action of the antihypertensive agent, prazosin. J Phar- macol Exper Ther 1977; 201:747-252.
- Bengtsson C, Johnsson G, Regårdh C. Plasma levelsand effects of metoprolol on blood pressure and heart rate in hypertensive patients after an acute dose and be- tween two doses during long-term treatment. Clin Pharmacol Ther 1975; 17: 400-408.
- Henry PD. Comparative pharmacology of calcium antagonists: nifedipine, verapamil and diltiazem. Am J Cardiol 1980; 46: 1047-1058.
- Jayo M, Cisneros F. Guide for the Care and Use of laboratory animals, Institute of Laboratory Animal Re- sources, Commission on Life Sciences, National Re- search Council, Washington, D.C.: National Academy Press; 1996.
- Figueroa-Valverde L, Díaz-Cedillo F, López-Ramos M, et al. Synthesis of pregnenolone-danazol-ethylendiam- ine conjugate: relationship between descriptors logP, π, Rm and Vm and its antibacterial activity in S. aureus andV. cholerae. Med Chem Res 2010, DOI: 10.1007/-s00044-010-9408-0.
- Ceballos G, Figueroa L, Rubio I, et al. Acute and Non- genomic Effects of Testosterone on Isolated and Per- fused Rat Heart. J Cardiov Pharmacol 1999; 33:691- 697.
- Figueroa-Valverde L, Díaz-Cedillo F, Diaz-Ku1 E, et al. Effect induced by hemisuccinate of pregnenolone on perfusion pressure and vascular resistance in isolatedrat heart. African J Pharmacy Pharmacol 2009; 3(5):234-241.
- Figueroa Valverde L, Ceballos-Reyes G, Díaz-Cedillo F, et al. Biological activity of progesterone-dihydro- pyridimidine derivative on perfusion pressure andcoronary resistance in isolated rat heart. African J Pharmacy Pharmacol 2010; 4 (4):170-177.
- Figueroa L, Díaz F, Camacho A, et al. Actividad indu- cida por androsterona y hemisuccinato de androsteronasobre la presión de perfusión y la resistencia vascular.Biomédica 2009; 29: 625-634.
- Drew G, Witing S. Evidence for a-adrenoceptor two in distinct vascular types of postsynaptic smooth muscle in vivo. Br J Pharmac 1979; 67: 207-215.
- Rajala G, Kolesari G, Kuhlmann R, et al. Ventricu- lar blood pressure and cardiac output changes in epi- nephrine- and metoprolol-treated chick embryos. Exp Teratol 1988; 38: 291-296.
- Hocht C, Opezzo J, Gorzalczany S, et al. Una aproximación cinética y dinámica de metildopa en ratas con coartación aórtica mediante microdiálisis. Rev ArgCardiol 1999; 67: 769-773.
- Ely D, Salisbury R, Hadi D, et al. Androgen receptor and the testes influence hypertension in a hybrid rat model. Hypertension 1991; 17: 1104-1110.
- Kumai T, Tanaka M, Watanabe M, et al. Influence of androgen on tyrosine hydroxylase mRNA in adrenal medulla of spontaneously hypertensive rats. Hyperten- sion 1995; 26: 208-212.
- Lilley J, Golden J, Stone R. Adrenergic regulation of blood pressure in chronic renal failure. J Clin Invest 1976; 57:1190-1200.
- Pham T, Robinson R, Danilo P, et al. Effects of gona-dal steroids on gender-related differences in transmural dispersion of L-type calcium current. Cardiovasc Res 2002; 53:752-762.