- Biomedical Research (2009) Volume 20, Issue 2
Acrolein induces unilateral hypertrophy and associated histopathalogical changes during germ cell differentiation in mature rat ovary
Ajit K Saxena*, Divya Singh and Gajendra SinghHuman Cytogenetic Laboratory, Centre of Experimental Medicine and Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- *Corresponding Author:
- Ajit K Saxena Human
Cytogenetics Laboratory
Centre of Experimental Medicine and Surgery
Institute of Medical Sciences
Banaras Hindu University Varanasi221 005, India
Accepted November 13 200
Abstract
Acrolein is an important chemical molecule of the global environment. It has dual importance as a by product of industrial wastes, automobile exhaust, and secondly an active metabolite of cyclophosphamide metabolic pathway. Acrolein is highly reactive unsaturated three carbon aldehyde. The importance of this chemical molecule increased several folds because of high reactivity; therefore, study has been designed with the aim to evaluate the effect of low dose (1mg/kg body wt. /day), continuous exposure (i.p.) of acrolein alone or with ascorbate (5 mg/kg body wt) for 10 days to rats .Reproductive performance of the treated rats were observed by serial mating experiments with opposite sex of the same strain. Infertility (33%) was observed when compared with controls. Most interesting finding is significant reduction (P<0.001) in the size of germ cell – populations i.e. primary, secondary and graafian follicles in medulla as well as in cortex region of the ovary as observed in experimental group. In another set of experimental group where antioxidant (ascorbic acid) was supplemented along with acrolein which shows regeneration of germ cells proliferation, suggesting ascorbate help to prevent cell-damage during cell-division in female gonads. The study was also further extended to observed genetic damage in term of chromosomal aberrations assay which reveals that significant changes (p < 0.05) were observed when compared with control groups.
Keywords
acrolein, hypertrophy, rat ovary, fertility and chromosomal aberration
Introduction
In-vivo acrolein is a by product of cyclophosphamide metabolic pathway and has been found to be formed from threonine by neutrophil myeloperoxidase at sites of inflammation [1,2]. Acrolein is highly toxic in nature and therefore improve the efficacy of cyclophosphamide therapy or to reduce the toxicity of the other xenobiotics using antioxidants. Acrolein, a wide spread environmental pollutant is found in all type of smoke and is formed in polluted air as a photochemical reaction product, although teratogenecity of this molecule are well documented in literature but lack on gonads [3]. Information regarding the effect of chemotherapeutic agent and its metabolites on female reproductive system is not well documented in literature, except few reports had been documented on devastation of oocytes and premature ovarian failure in women [4,5,6]. In the experimental study on mice shows significant damage of oocytes and follicular cells has been reported [7]. In ambient air acrolein is estimated at only 0.04 to 0.08 ppm, but the concentration is increased up to 90ppm in vapor phase of cigarette smoke [8], in auto mobile exhaust [9]. Ubiquitous presence in the environment and over reactivity of acrolein becomes highly toxic in nature. In-vitro concentrations of 25 to 100 uM are lethal to pulmonary artery endothelial cells, bronchiolar epithelial cells and both bronchial and cardiac fibroblasts [10,11,12,13]. Acrolein induces oxidative stress because of loss of glutathione activity (GSH) due to alkyaltion reactions at various nucleophilic sites in a cell particularly in the nucleus when exist in higher concentration. At low doses acrolein inhibits cell proliferation without causing cell-death and may enhance apoptosis from secondary toxins. Alternately, in -vitro study indicates that the direct alkylation of DNA by acrolein at low doses seems to be insignificant in human lung adenocarcinoma cells [14,15,16]. Large number of genes and its transcription factors (protein) have been identified to affect cell – division and apoptosis [17]. The exact mechanism of acrolein mediated cellular, specific point of chromosomal breaks and exchanges has been poorly defined in literature. It has also been observed in patient of diabetic, nephropathy and Alzheimer’s disease with increase lipid peroxidation [18,19].However, the mechanism of action of acrolein has not been known satisfactorily whether acrolein itself or its metabolites are responsible for germ – cell mediated toxicity with special reference to mature female gonads, although it induces spectrum of malformations in rat [20]. Hence, the interest has been generated several folds to monitor the influence of acrolein on ovary with or without use of antioxidant on gonadal dysfunction using rat as a putative animal model.
Materials and Methods
Acrolein was purchase from E. Merck (Germany). Female C.F strain of albino rats (n=30) of approximately 200-250 gms were obtained from central animal house of Institute of Medical Sciences, BHU, used both for experimental as well as control groups in present study. Food and water were provided ad libitum and animals were maintained on a 12L: 10D cycle. The animals were divided into three groups and each group contain ten rats:- Group-I acts as controls, Group-II: Experimental group includes where acrolein was administered 5 ugm/day (i.p.) for 10 days continuously, while Group-III where animals received both acrolein and antioxidant using ascrobat (5gm/kg body wt/day.). At the end of the day, the half of the animals (n=5) were sacrificed for histological and cytogenetic study while half of the animals (n=5) were used to evaluate the reproductive performance of the females using serial matting experiment between normal healthy male rats of the same strain and compared with controls.
For histological studies ovaries were removed; washed and separate (right and left) weight was taken from all experimental groups along with controls. Tissues were fixed in 10% formalin and stained with H/E staining using routine procedure. Every alternative section of 6μm were taken into account for microscopic examination such as morphological features and diameter of the follicles using micrometer disc fitted in objective of the microscope which contains 100 equally spaced division, marked 0-10. One division of the stage micrometer equals to 10 micrometer or 0.01 micrometer.
The cytogenetics studies were carried out using bone – marrow cells collected from the same experimental and controls groups by the procedure of Saxena (21) for direct preparation of chromosomal analysis from each data point. For scoring of chromosomal aberrations, well spread metaphase plates were selected for chromosomal studies after using 5% Gimsa as stain reagent.
Statistical Analysis
Chi Square test was used to observed the significant differences (P – value) between experimental and control groups by the method of Snedecor and Cochran [22].
Results and Discussion
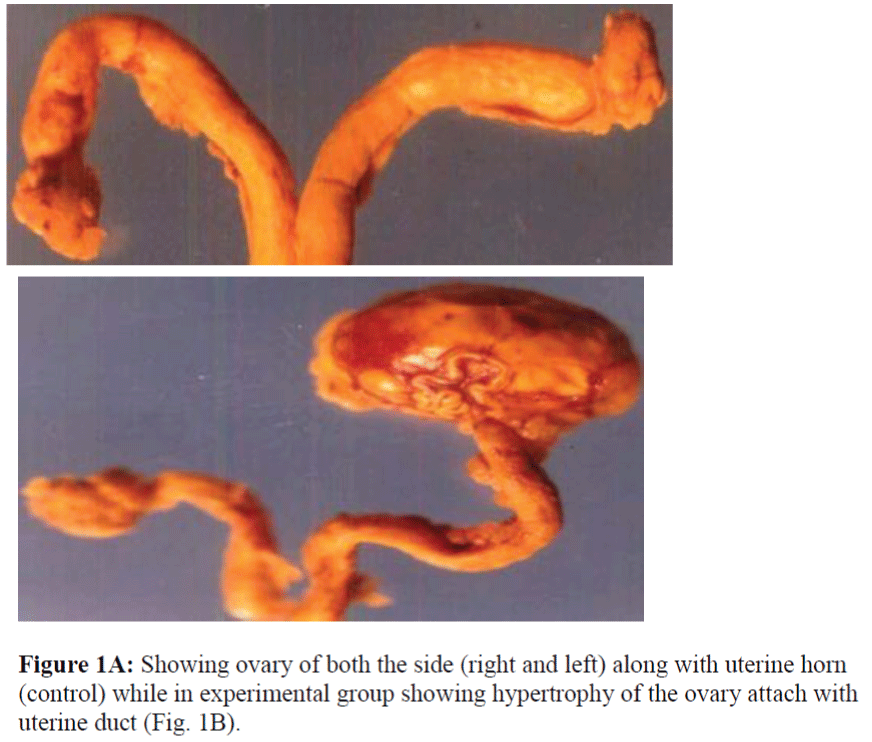
The morphological (qualitative) discrepancies were observed such as significant reduction of the size of the follicles after continous low doses exposure of acrolein up to ten days to the female rats, interestingly in one case “unilateral hypertrophy” of ovary on left side was seen while on other side normal in appearance in experimental groups of the same animal when compare with controls as shown in Fig.1A and B. Although, morphologically, ovary appears an almond-shaped, containing a rich vascular bed within a cellular loose connective tissue as seen in control groups.
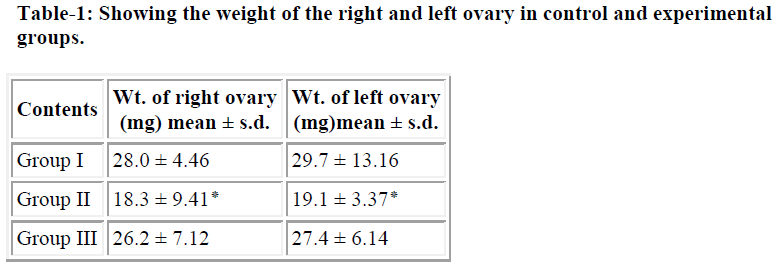
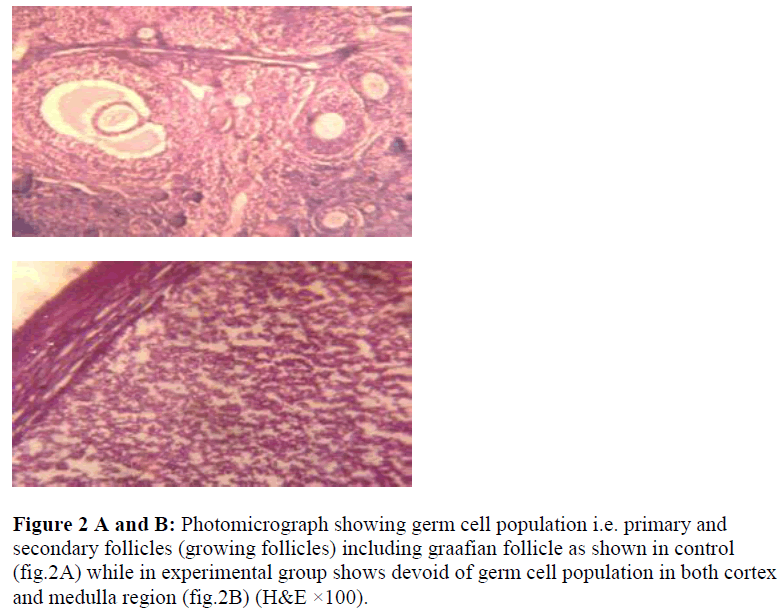
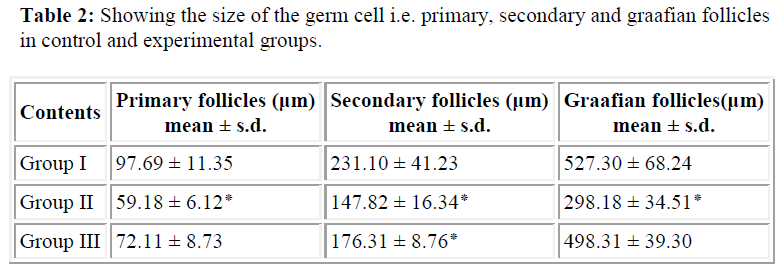
Table 1 shows the weight of the right and left ovaries in both experimental groups were observed and compared with control group which revealed significance differences may be because of cytotoxic nature of the acrolein In histological finding using routine H&E staining of the ovarian sections (T.S.) reveals well defined population of germ cells (follicles) at various stages of development are embedded in stroma region of the ovary with loose connective tissue, while numerous primary follicles appear in the cortical region as seen in control group (Fig.2A). The mature follicle (Graafian follicle) is appears with large cavity full of acidophilic fluid although, intermediate follicles (secondary) are also visible in deep part of the cortex region separated by surrounding ovarian stroma. In experimental groups the ovarian tissue shows complete disappearance of germ cell population in cortical and medullary region when compare with controls (Fig. 2B) due to cytotoxic nature of the acrolein which inhibit DNA as well as protein synthesis [23]. The size of the proliferating germ cells (primary, secondary and graafian follicles ) were also effected in experimental groups, although significance difference (p<0.001) were observed because of either the metabolization of acrolein into various metabolites or due to low dose effect in the mature gonads (Table-2). Interestingly, the size of the mature follicle increased where the antioxidant were administered suggesting ascorbate either detoxifying the effect of acrolein or help for regeneration of proliferating germ cells in female gonads. In another set of experimental group (only acrolein administered) which shows significant differences in term of chromosome aberrations including chromosome break (both chromosome and chromatid type), gap and fragmentation are the significant finding were observed although similar findings of chromosome alterations were also observed by the same authors Saxena and Singh [24] using cyclophosphamide administration in-vivo. The frequencies of chromosomal aberrations like break/gaps and exchanges were significantly decreased where ascorbate was used as an antioxidant suggesting ascorbate prevent genetic damage. The distribution of breaks and exchange points along the chromosome strongly indicate that heterochromatic areas are not the site of preferential “attack” by the chemical (acrolein). Drug likes cyclophosphamide or its active metabolite- acrolein, does bind to purines and heterochromatic regions have been suggested to be rich in guanine-cytosine content, it does not mean that the chromosome-breaking action of such chemicals is through their influence in guanine-cytosine rich regions. Acrolein is negative regulators of the cell-cycle and p53 gene product and expression, while arresting cell-division for rapidly dividing cells (primary and secondary follicles) of the ovary in either G1 or G2 phases of the cell – cycle probably due to mutagenic nature [25].
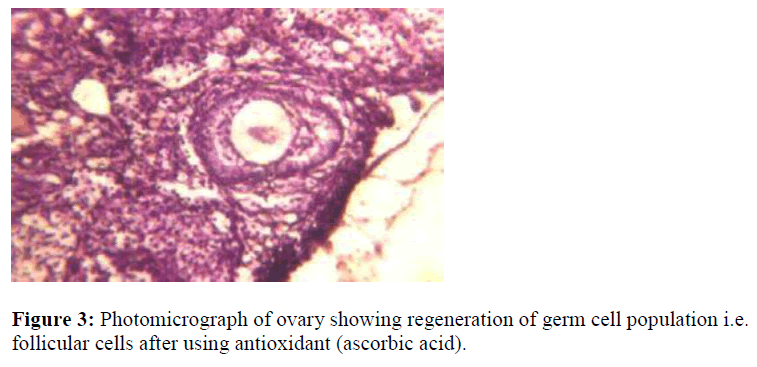
In another set of experimental group where antioxidant (Ascorbic acid) was given along with acrolein shows very interesting findings i.e. regeneration of germ – cell proliferation was observed as shown in Fig. 3 result increase in population of germ cell. This may be either due to detoxification of acrolein by conjugation with glutathione (GSH) in the cell or due to low dose exposure of acrolein may cause inhibit of cell proliferation temporally as observed in the present study without causing cell- death. The cytotoxic nature is caused by alkylation reaction of the acrolein with DNA and protein inhibits cellular damage in cells [26], while high dose of acrolein induces oxidative stress either due to loss of glutathione (GSH) or due to alkyation reaction in a cell, particularly in nucleus. Present study suggests that ascorbate helps for the protection of damage germ cell by slowing oxidative process by scavengering ROS molecules that begin in the absence of adequate GSH during stress [27]. The reproductive performance shows 33% infertility when compare with control groups because of cytotoxic and mutagenic nature of the compound induces degeneration of growing follicles in mature female gonads.
Conclusion
Hence, the present study has been designed using rat as putative animal model not possible in case of human being to concluded with the remark that acrolein – an environmental pollutant – is highly toxic in nature which causes the following changes-1) degeneration of germ cells proliferation in rat ovary, 2) regeneration of germ cell population after use of antioxidant (ascorbic acid), 3) changes in chromosomal features in bone-marrow cells, and 4) alteration in reproductive performance.
Acknowledgement
AKS is thankfully acknowledged to C.S.I.R., New Delhi.
References
- Alacron RA, Meienhofer J. Formation of the cytotoxic aldehyde acrolein during in-vitro degradation of cyclophosphamide. Nature 1971; 233, 250-252.
- Anderson MM., Hazen SL, Hsu FF, Heinceke JW. Human neutrophils employ the myeloperoxidase- hydrogen peroxide-chloride system to convert hydroxy- amino acid into glycoaldehyde, 2-hydropropanal, and acrolein: A mechanism for the generation of highly reactive alpha-hydroxy and alpha, beta-unsaturated aldehyde by phagocytes at sites of inflammation. J Clin Invest 1977; 99, 424-432.
- Slott VL, Hales BF. Teratogenicity and embryolethality of acrolein and structurally related compounds in rats. Teratology 1985; 32, 65-72.
- Miller JJ, Williams GF, Leissing JC. Multiple late complication of therapy with cyclophosphamide, including ovarian destruction. Am J Med 1971; 50, 530-535.
- Wame GI, Fairley KF, Hobbs JB, Martine FI. Cyclophosphamide induced ovarian failure. N Eng J Med 1973; 289, 1159-1162. Effect of acrolein on fertility and cellular changes in rat ovary.
- Ross DP, Davis TE. . Ovarian function in patients receiving adjuvant chemotherapy for breast cancer. Lancet 1977; 1, 1174-1176.
- Ataya KM, Valeriote FA, Ramahi- Ataya A.J. Effect of cyclophosphamide on the immature rat ovary. Cancer Res 1989 ;49(7), 1660-1664.
- Costa DL, Amdur MO. Air pollution. In Casarett and Doul’s Toxicology 5th ed. 857-882, 1996.
- Tonimoto M, Uehara H, Detection of acrolein in engine exhaust with microwave capacity spectrometer of spark voltage type. Environ Sci Technol 1975; 9, 153- 154.
- Kachel DL, Martin Wl II . Cyclophosphamide induces lung toxicity. Mechanism of endothelial cell injury. J Pharmacol. Exp Ther 1994; 268, 42-46.
- Patel JM, Block ER. Acrolein- induced injury to cultured pulmonary artery endothelial cells. Toxicol Appl Pharmacol 1993; 122, 46-53.
- Grafstrom R, Dypbukt JM, Willey JC, Sundqvist K, Edman C, Atzori L, Harris CC. Pathobiological effects of acrolein in cultured human bronchial epithelial cell. Cancer Res 1988; 48, 1717-1721.
- Torasson M, Luken ME, Breitenstein M, Krueger JA, Biagini R.E. Comparative toxicity of allylamine and acrolein in cultured myocytes and fibroblasts from neonatal rat heart. Toxicology 1989; 56, 107-117.
- Horton ND, Maniya BM, Kehrer JP. Relation ship between cell density, glutathione and proliferation of A549 human lung adenocarcinoma treated with acrolein. Toxicology 1997;122, 111-122.
- Ramu K, Perry CS, Ahmed T, Pakenham G, Kehrer JP. Studies on the basis for the toxicity of acrolein mercapturates. Toxicol Appl Pharacol 1996; 140, 487-498.
- Rudra PK, Krokan HE. Acrolein cytotoxicity and glutathione depletion in n-3 fatty acid sensitive and resistance human tumors cells. Anticancer Res 1999; 19, 461-469.
- Horton ND, Biswal SS, Corrigan LL, Bratta J, Kehrer JP. Acrolein causes Iκβ – independent decreases in NF-κβ activation inhuman lung adenocarcinoma (A549) cells. J Biol Chem 1999; 9200-9206.
- Suzuki D, Mitaya T. Carbonyl stress in the pathogenesis of diabetic nephropathy. Intern Med 1999; 38, 309-314.
- Calingasan NY, Uchida K, Gibson GE. Protein bounded acrolein: A novel marker of oxidative stress in Alzheimer’s disease. J Neruochem 1999; 72, 751-756.
- Hales BF. Comparison of the mutagenicity and teratogenicty of cyclophosphamide and its active metabolites, 4-hydroxy cyclophosphamide, phosphoramide mustard and acrolein. Cancer Res 1982;42, 3016-3021.
- Saxena AK. Changes in the frequency of chromosomal aberrations in three different tissues (liver, testis and embryonic tissue), after antenatal exposure of the fetus at various intervals in rat to cyclophosphamide. Proc Natl Acad Sci (India) 1994; 64(B), 27-34.
- Snedecor GW, Cochran WG. Statistical methods, 6th ed. Iowa State University Press, Ames, IO, 1967.
- Munsch N, Recondo AM, Frayssinet C. Effect of acrolein on DNA synthesis in- vitro. Febs Letters 1973; 30, 286-290.
- Saxena AK, Singh G. Aneuploidy induced by cyclophosphamide in developing rat testis. In Vitro Cell and Dev Biol. Animal 1998; 34(1), 11-13.
- Hainaut P, Milner J. Redox modulation of p53 conformation and sequence specific DNA binding invitro. Cancer Res 1993; 53, 4469-4473.
- Wheeler G P. Studies related to the mechanism of action of cytotoxic alkylating agent: a review. Cancer Res 1962; 22, 651-688.
- Esposito F, Cuccovillo F, Morra F, Russo T, Cimino F. DNA binding activity of glucocorticoid receptor is sensitive to redox changes in intact cells. Biochem Biophys Acta 1995; 1260, 308- 314.