Research Article - Journal of Orthopedic Surgery and Rehabilitation (2017) Volume 1, Issue 3
Accelerated callus formation and fracture union in patients with femoral or tibial shaft fracture and traumatic head injuries.
Hee-Gon Park*, Yong-Eun Shin
Department of Orthopedic Surgery, Dankook University College of Medicine, Cheonan, Korea
- *Corresponding Author:
- Hee-Gon Park
Department of Orthopedic Surgery, Dankook University College of Medicine, Cheonan, Korea
Tel: +82 1899-3700
E-mail: 120826@dkuh.co.kr
Accepted date: September 19, 2017
Citation: Park HG, Shin YE. Accelerated callus formation and fracture union in patients with femoral or tibial shaft fracture and traumatic head injuries. J ortho Rehab Surg. 2017;1(3):1-4
Abstract
Aim and Purpose: The purpose of this study is to reveal whether the patients with femoral or tibial shaft fracture and traumatic head injuries accelerates callus formation, as well as fracture union periods. In advance, it allows orthopedic surgeons to predict earlier fracture union period and reassure the patients and their family.
Methods: 291 patients who underwent closed reduction and intramedullary nail fixation for the femoral or tibial shaft fractures and followed up at least 1 year were analyzed retrospectively. Callus formation and fracture union were quantified by serial radiographic images. The level of consciousness and Glasgow Coma Scale, location and severity of head injuries were evaluated by neurosurgeon at the time of admission. Cases were subdivided into two groups; group A consisted of the patients who had concomitant traumatic head injuries, and group B consisted of the patients who had not concomitant traumatic head injuries.
Results: The mean callus ratio and volume were significantly greater in group A than group B. The fracture union period was shorter in group A than group B. There was no statistically difference for gender and age between two groups. The level of consciousness, Glasgow coma scale, location and severity of head injuries, and experience of neurosurgical intervention were not significant factors in predicting the rate of bone healing and the extent of callus formation in group A.
Conclusion: Femoral or tibial shaft fractures with concomitant traumatic head injuries regardless of the severity or location of injuries were demonstrated to enhance bone healing with increased callus formation and decreased fracture union period. Orthopedic surgeons should evaluate traumatic head injuries prudently with assistance of neurosurgeons if needed even though the patient was alert consciousness state.
Keywords
Femoral fracture, Tibial fracture, Traumatic head injury, Bone healing, Callus formation.
Introduction
Fractures of the femoral or tibial shaft are relatively common injuries. Previous reported incidence was 21-37.1 per 100,000 person-years [1,2] and 16.9 per 100,000 personyears, respectively [3]. Its incidence has been increased with development of various transportation and numerical increment of traffic accidents [4,5]. Consequently, femoral or tibial shaft fractures with traumatic head injuries increased with increasing high energy trauma cases. The association between traumatic head injury and enhanced osteogenesis has been debated. Those studies documented increased callus formation and shorter fracture union period were found in patients with severe brain injury and concomitant long bone fractures [6-8].
The purpose of this study is to reveal whether the femoral or tibial shaft fracture patients with traumatic head injuries accelerates callus formation, as well as fracture union periods. In advance, it allows orthopedic surgeons to predict earlier fracture union period and reassure the patients and their family.
Materials and Methods
Patient selection
The patients whose fracture were located in femoral or tibial diaphysis and treated with closed reduction and reamed antegrade intramedullary nail fixation with conventional technique in the author’s institution between March 2006 and March 2014 were included. Patients who were not followed up with serial clinical examinations and radiographic evaluations on a minimum of four different occasions with at least 12 months follow up were excluded. Patients with evidence of gross deformity, remote fractures or surgical history around fracture site, prior nervous system problem were excluded. After applying these inclusion and exclusion criteria, 291 patients were enrolled.
The patients were divided into 2 groups. Group A consisted of the patients who had concomitant traumatic head injuries regardless of severity, and group B consisted of the patients who had not concomitant traumatic head injuries. In group A, there were 25 males and 4 female patients, with a mean age of 52.2 ± 18.9 years (range, 18-79 years). In group B, there were 228 males and 34 female patients, with a mean age of 48.7 ± 15.5 years (range, 18-77 years). There was no statistically significant difference for gender (p=0.436), and age (p=0.542) between the two groups (Table 1).
| Variables | Total | Group A | Group B | P-value |
| Case | 391 | 29 | 362 | |
| Gender (M:F) | 323:68 | 25:4 | 298:64 | 0.436 |
| Age, years | 44.9 ± 17.1 (18-79) |
52.2 ± 18.9 (18-79) |
48.7 ± 15.5 (18-77) |
0.542 |
Table 1: Demographic data.
Neurologic evaluation
The level of consciousness and Glasgow Coma Scale [9] were evaluated by neurosurgeon at the time of admission. The location and severity of head injuries were determined by 3-dimensional computed tomography and were scored with the Marshall computed tomography classification system [10]. It was also done by neurosurgeon at that time. The patients whose concomitant traumatic head injuries were severe enough to be observed intensively were admitted and cared in intensive care unit (ICU), and orthopedic surgery were undergone after patients’ conditions were recovered thoroughly with neurosurgeon’s decision.
Radiologic evaluation
The femoral or tibial shaft fractures were imaged by conventional radiographs in anteroposterior (AP) and lateral projections. Serial clinical examinations and radiographic evaluations were checked for minimum of four different occasions with at least 12 months after surgery.
The image of each patient was accessed using the picture archiving and communication system (PACS-ViewRex3; Techheim, Seoul, Korea). The size, homogeneity, density of bony callus formation, position of the bone fragments, position of intramedullary nails, and evidence of union were visually estimated by the simple radiograph images.
Callus formation and callus size were quantified with use of the method previously described by Spencer [11]. Shortly, the ratio was calculated with use of the widest callus diameter which measured at 90° to the long axis of the bone and the diameter of the normal adjacent diaphysis on the anteroposterior and lateral radiographs. The highest ratio calculated during fracture healing was used for analysis. Additionally, the volume of callus was calculated using the Perkins volume formula (2πR1(R2-R1) L, where R1=Radius of femur, R2=Radius of callus, L=Length of callus) [7].
Radiographic union was determined traditionally as cortical bridging by callus and the lack of a fracture line [12]. We used radiographic union scale in tibial fractures (RUST) score [13] and the score of 9 or over considered fracture union according to the literature by Litrenta et al. [14] Since the radiological examination could be influenced by inter- and intra-observer reliability, 2 blinded observers repeated the measurements 2 weeks apart.
Statistical analysis
Independent t-test was used to compare age, gender, quantity of the callus formation and periods to achieve bone union between two groups. Univariate regression analysis was used to evaluate the relation between Glasgow-coma scale and quantity of the callus formation, periods to achieve bone union in group A. The tests mentioned above were performed using IBM SPSS ver. 21.0 (IBM Co., Armonk NY, USA) and the p values of<0.05 were considered statistically significant.
The weighted kappa(k) coefficient was used to estimate the inter- and intra-observer reliability. Inter- and intra-observer reliability was classified according to the k coefficients: “slight agreement”, 0.00-0.20; “fair agreement”, 0.21-0.40; “substantial agreement”, 0.61-0.80 and “almost perfect agreement”, 0.81- 1.00.
Results
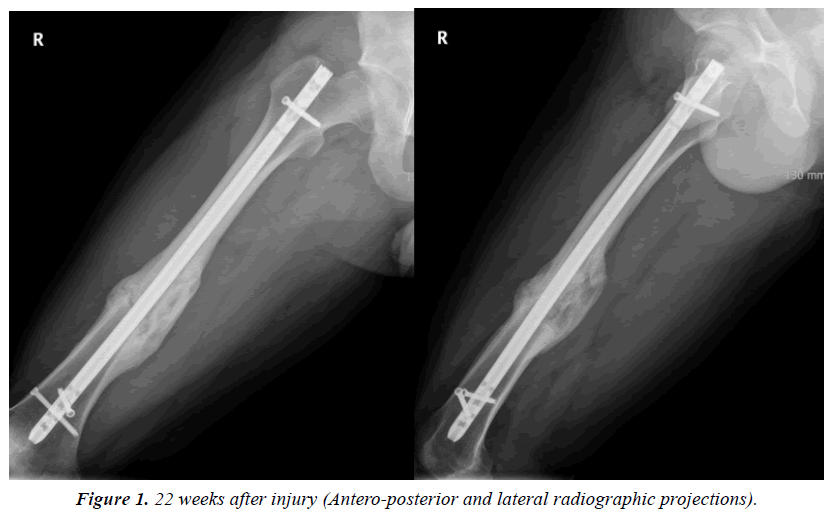
The mean callus ratio of femoral or tibial shaft fractures was considerably greater on both the anteroposterior and the lateral radiographs in group A than in group B (1.58 and 1.21, respectively, on the anteroposterior radiograph and 1.62 and 1.19 on the lateral projection [p<0.01]; Table 2 and Figure 1). The mean callus volume was significantly greater in group A than in group B (57.42 and 33.18, respectively [p<0.01]). The fracture union period was shorter in group A than in group B (14.7 and 22.3 weeks, respectively [p=0.01]). The duration of hospital stay was longer in group A than in group B (5.2 and 2.3 weeks, respectively [p<0.01]).
| Variables | Group A | Group B | P-value | |
|---|---|---|---|---|
| Mean callus ratio | AP | 1.8 | 1.3 | <0.01 |
| Lateral | 1.7 | 1.3 | <0.01 | |
| Mean callus volume, cm3 | 57.42 | 33.18 | <0.01 | |
| Fracture union period, weeks | 23.7 | 32.3 | 0.01 | |
| Duration of hospital stay, weeks | 5.2 | 2.3 | <0.01 | |
Table 2: Comparison of group A and group B.
Categorizing according to the severity of traumatic head injuries in group A, there were 6 cases of epidural hemorrhage, 9 cases of subdural hemorrhage, 7 cases of sub-arachnoidal hemorrhage, 2 cases of intracerebral hemorrhage, and 5 cases of cerebral contusion. There was no significant difference between the severity of traumatic head injuries and callus formation or bone union (p=0.528, 0.387, respectively). According to the level of consciousness, there were 11 cases of alert, 7 cases of drowsy, 4 cases of stupor, 4 cases of semicoma, 3 cases of coma. There was no significant difference between the level of consciousness and callus formation or bone union (p=0.446, 0.375, respectively). 13 cases of group A undergone operative neurosurgical intervention, and there was no significant difference between the experience of operative neurosurgical intervention and callus formation or bone union (p=0.548, 0.726, respectively). There was no correlation between the Glasgow-coma scale and callus formation or bone union by regression analysis (p=0.625, 0.587, respectively).
Inter- and intra-observer reliability was “almost perfect agreement”, with 0.87 and 0.82 of weighted kappa(k) coefficient values.
Discussion
Patients with traumatic head injuries and femoral or tibial shaft fractures were enhanced fracture healing with increased callus formation by mean callus ratio and mean callus volume on both anteroposterior and lateral radiographic projections and shorter fracture union period in the present study. These results are consistent with previous clinical studies that have described this phenomenon both in femoral and other long bone fractures [15-17] The exact pathophysiologic mechanism responsible for this phenomenon remains controversial up to date. However, there is a consensus regarding enhanced fracture-healing in patients with associated traumatic head injuries.
The authors revealed that age, gender, severity of traumatic head injuries, level of consciousness, experience of operative neurosurgical intervention, and Glasgow-coma scale were not statistically significant factors in predicting the rate of bone healing and extent of final callus formation and these results are also consistent with previous studies [8,18].
In the last few years, very little is revealed of the complex mechanism by which cytokines, chemokines, hormones, and growth factors influence the signaling pathway leading to accelerated fracture healing [19]. The studies indicated that both serum and cerebrospinal fluid from patients with fractures and concomitant traumatic brain injury have osteoinductive potential. It also seems to be a consensus that these osteoinductive factors are released from the injured brain and from their spread in the body and to the fracture sites. Gautschi et al. [20] showed that the expression of osteoblastic markers (alkaline phosphatase (ALP), runtrelated transcription factor 2 (RUNX-2), cathepsin K (CATK), and serine protease 7(SP7)) enhanced fracture healing in concomitant traumatic brain injury patients, and SP 7 and CATK is more specific to the relationship between traumatic brain injury and enhanced fracture healing. Cadosch et al. [8] demonstrated that the patients with a severe brain injury release unknown humoral factors into the blood circulation that enhance and accelerate fracture healing. The leptin and calcitonin gene-related peptide (CGRP) also seems to play a role in the healing of fractures in patients with traumatic head injuries. Wei et al. [21] studied about serum leptin levels and leptin expression in callus cells with increased callus formation. They suggested a relationship between hypothalamic damage and reduced inhibition of peripheral leptin secretion and increased callus formation. Zhang et al. [22] showed the link between increased leptin levels and an enhanced expression of CGRP and its hemangiectasic role at the fracture site and increased fracture healing tendency.
Limitations
Limitations of this study should be addressed. First, this is a retrospective study, and thus control group was not selected properly. Second, subjects were limited to those with at least 12 months follow-up was done, it might lead to a selection bias. Third, although radiologic evaluations were performed quite well, biochemical analyses were not performed despite several literatures which argued that numerous factors affect fracture healing in traumatic head injury patients. Finally, radiologic evaluations were not conducted uniformed periods in each patient.
Further studies are needed to prove the hypothesis of accelerated fracture healing in patients with concomitant traumatic head injuries with a large prospective design. The research on the pathophysiological relationship between traumatic head injuries and callus formation should also be elaborated to reveal possible pathways.
Conclusion
Femoral or tibial shaft fractures with traumatic head injuries regardless of the severity or location of injuries demonstrated enhanced fracture healing with increased callus formation and decreased fracture union period. Orthopedic surgeons should evaluate traumatic head injuries prudently with assistance of neurosurgeons if needed even though the patient was alert consciousness state.
References
- Enninghorst N, McDougall D, Evans JA, et al. Population-based epidemiology of femur shaft fractures. J Trauma Acute Care Surg. 2013;74(6):1516-20.
- Arneson TJ, Melton LJ, Lewallen DG, et al. Epidemiology of diaphyseal and distal femoral fractures in Rochester, Minnesota, 1965-1984. Clin Orthop Relat Res. 1988;(234):188-194.
- Larsen P, Elsoe R, Hansen SH, et al. Incidence and epidemiology of tibial shaft fractures. Injury. 2015;46(4):746-50.
- Rastogi S, Wild BR, Duthie RB. Biomechanical aspects of femoral fractures in automobile accidents. J Bone Joint Surg Br. 1986;68(5):760-6.
- Courtney PM, Bernstein J, Ahn J. In brief: Closed tibial shaft fractures. Clin Orthop Relat Res. 2011;469(12):3518-21.
- Giannoudis PV, Mushtaq S, Harwood P, et al. Accelerated bone healing and excessive callus formation in patients with femoral fracture and head injury. Injury. 2007;38(10):1224.
- Perkins R, Skirving AP. Callus formation and the rate of healing of femoral fractures in patients with head injuries. J Bone Joint Surg Br. 1987;69(4):521-4.
- Cadosch D, Gautschi OP, Thyer M, et al. Humoral factors enhance fracture-healing and callus formation in patients with traumatic brain injury. J Bone Joint Surg Am. 2009;91(2):282-8.
- Teasdale G, Jennette B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81-4.
- Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9 Suppl 1:S287-92.
- Spencer RF. The effect of head injury on fracture healing. A quantitative assessment. J Bone Joint Surg Br. 1987;69(4):525-28.
- Whelan DB, Bhandari M, McKee MD, et al. Interobserver and intraobserver variation in the assessment of the healing of tibial fractures after intramedullary fixation. J Bone Joint Surg Br. 2002;84(1):15-8.
- Whelan DB, Bhandari M, Stephen D, et al. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma. 2010;68(3):629-32.
- Litrenta J, Tornetta P, Mehta S, et al. Development of radiographic healing: an assessment of consistency using RUST and modified RUST in metadiaphyseal fractures. J Orthop Trauma. 2015;29(11):516-20.
- Newman RJ, Stone MH, Mukherjee SK. Accelerated fracture union in association with severe head injury. Injury. 1987;18(4):241-6.
- Garland DE, Todler L. Fractures of the tibial diaphysis in adults with head injuries. Clin Orthop Relat Res. 1980;150:198-202.
- Morley J, Marsh S, Drakoulakis E, et al. Does traumatic brain injury result in accelerated fracture healing. Injury. 2005;36(3):363-8.
- Yang TY, Wang TC, Tsai YH, et al. The effects of an injury to the brain on bone healing and callus formation in young adults with fractures of the femoral shaft. J Bone Joint Surg Br. 2012;94(2):227-30.
- Hofman M, Koopmans G, Kobbe P, et al. Improved fracture healing in patients with concomitant traumatic brain injury: proven or not? Mediators Inflamm. 2015;204842.
- Gautschi OP, Cadosch D, Frey SP, et al. Serum-mediated osteogenic effect in traumatic brain-injured patients. ANZ J Surg. 2009;79(6):449-55.
- Wei Y, Wang L, Clark JC. Elevated leptin expression in a rat model of fracture and traumatic brain injury. J Pharm Pharmacol. 2008;60(12):1667-72.
- Zhang D, Zhang P, Wang Y. The influence of brain injury or peripheral nerve injury on calcitonin gene-related peptide concentration variation and fractures healing process. Artf Cells Blood Substit Immobil Biotechnol. 2009;37(2):85-91.