Case Report - Annals of Cardiovascular and Thoracic Surgery (2018) Volume 1, Issue 3
Abrupt onset of pulmonary hypertension and atypical haemolytic-uremic syndrome in a young child; diagnosis and successful treatment of rare metabolic disorder.
Sacchini M1, Capirchio L2, De Simone L2*, Brambilla A2, Capponi G2, Roperto R3, Bennati E2, Spaziani G2, Porcedda G2, Calabri GB2, Assanta N4 and Favilli S2
1Nephrology and Dialysis Unit, Anna Meyer Children’s University Hospital, Florence, Italy
2Pediatric Cardiology Unit, Anna Meyer Children’s University Hospital, Florence, Italy
3Nephrology and Dialysis Unit, Anna Meyer Children’s University Hospital, Florence, Italy
4Cardiology Unit, Heart Hospital G. Monasterio Tuscany Foundation, Pisa, Italy
- *Corresponding Author:
- Luciano De Simone, MD
Paediatric Cardiology Anna Meyer Children Hospital Viale Pieraccini 24, Florence, Italy
Tel: +39 055 566 2498
E-mail: l.desimone@meyer.it
Accepted date: September 26, 2018
Citation: Sacchini M, Capirchio L, De Simone L, et al. Abrupt onset of pulmonary hypertension and atypical haemolytic-uremic syndrome in a young child; diagnosis and successful treatment of rare metabolic disorder. Ann Cardiovasc Thorac Surg 2018;1(3):52-56.
DOI: 10.35841/cardiovascular-surgery.1.3.52-56
Visit for more related articles at Annals of Cardiovascular and Thoracic SurgeryKeywords
Pulmonary hypertension, Thrombotic microangiopathy, Inborn errors in metabolism, Paediatric cardiology
Case Description
The primary subject matter of this case concerns: paediatric cardiology, pulmonary hypertension. Secondary issues examined include: Cobalamin C deficiency, thrombotic microangiopathy. The case has a difficulty level of seven: appropriate for doctoral students. The case is designed to be taught in two class hours and is expected to require four hours of outside preparation by students.
Case Synopsis
The enclosed case report exemplifies the problem of differential diagnosis while managing pulmonary hypertension in children. In the present case, the concomitant development of pulmonary hypertension and atypical haemolytic uremic syndrome suggested the diagnosis of underlying metabolic disorder, i.e. methylmalonic acidemia and homocystinuria due to Cobalamin C deficiency. Early identification of a treatable cause of pulmonary hypertension is mandatory, as to ensure the optimal final outcome and long-term survival to affected patients.
Case Report
A healthy 2-years boy was admitted to our hospital due to severe respiratory distress, with tachypnea (RR >60/min) and desaturation (blood oxygen saturation <85%). Within few hours from admission, severe systemic hypertension and anasarca appeared, requiring anti-hypertensive and diuretic treatment. Cardiologic evaluation excluded congenital heart diseases, whereas it revealed concentric left ventricular hypertrophy and signs of severe Pulmonary Artery Hypertension (PAH), with peak tricuspid regurgitation velocity >5 m/s. Mechanical respiratory support and phosphodiesterase-5 inhibitor treatment were promptly started, with initial stabilization of clinical parameters. Laboratory work-up documented mild anaemia, thrombocytopenia, increased blood creatinine level, reduced C3 and C4 complement fractions and positive haemolytic tests, suggesting the diagnosis of Haemolytic Uremic Syndrome (HUS) (Table 1).
| ONSET | LAST FOLLOW-UP | NORMAL VALUES | |
|---|---|---|---|
| WBC (x103 /microL) | 12 | 12,8 | 5-15 |
| RBC (x106 /microL) | 3,22 | 4,56 | 4,10-5,5 |
| Hb (g/dl) | 8,9 | 13,4 | 12-14 |
| MCV (fl) | 87,3 | 85,6 | 75-85 |
| RDW (%) | 23,1 | 12,7 | 11,6-16,5 |
| PLT (x103 /microL) | 61 | 347 | 130-400 |
| Creatinine (mg/dl) | 0,93 | 0,35 | 0,1-0,36 |
| Blood urea (mg/dl) | 91 | 16 | 9-22 |
| Calcium (mg/dl) | 9,1 | 9,6 | 9,2-10,3 |
| LDH (UI/L) | 1390 | 292 | 192-321 |
| Aptoglobine (mg/dl) | <7,75 | 62 | 30-200 |
| Total bilirubin (mg/dl) | 0,9 | 0,05 | 0,05-0,4 |
| Total protein (g/dl) | 5,1 | 7,4 | 6,1-7,5 |
| Albumin (serum) (g/dl) | 3 | 3,9 | 3,5-4,5 |
| Proteinuria (mg /24h) | 202,6 | 75,8 | <150 |
| Total urinary proteinuria (mg/L) | 3247 | 94 | <100 |
| Albumin (urine) (mg/L) | 2510 | 31,8 | <25 |
| IgG (Urine) (mg/L) | 112 | 4,6 | <5 |
| A1 microalbumin | 47,3 | <7,81 | <10 |
| C3c (mg/dl) | 81 | 107 | 90-180 |
| C4 (mg/dl) | 19 | 26 | 10-40 |
| IgG (mg/dl) | 223 | 949 | 432-1100 |
| IgA (mg/dl) | <7,83 | 55,9 | 30-125 |
| IgM (mg/dl) | 72 | 52 | 40-120 |
| Vitamin B12 (pg/ml) | 605 | >6000 | 160-800 |
| Folate (ng/ml) | 4,5 | 132 | 3-15 |
| Total cholesterol (md/dl) | 247 | 177 | 45-210 |
| Triglycerides (mg/dl) | 282 | 121 | 53-258 |
| ADAMTS-13 activity (%) | 80 | - | 50-150 |
| ADAMTS-13 Inhibitors (U/ml) | 1 | - | 0-15 |
| Homocysteine (micromol/L) | 74 | 8,7 | <15 |
| Propyonilcarnitine (C3 micromol/L) | 5,64 | 0,89 | <0,86 |
| Metylmalonic acid (serum) (micromol/L) | 138 | 1,9 | <1 |
| Metylmalonic acid (urine) (mM/mol) | 919 | 2 | <2 |
Table 1: Laboratory data of reported patient ad admission and at last available follow-up.
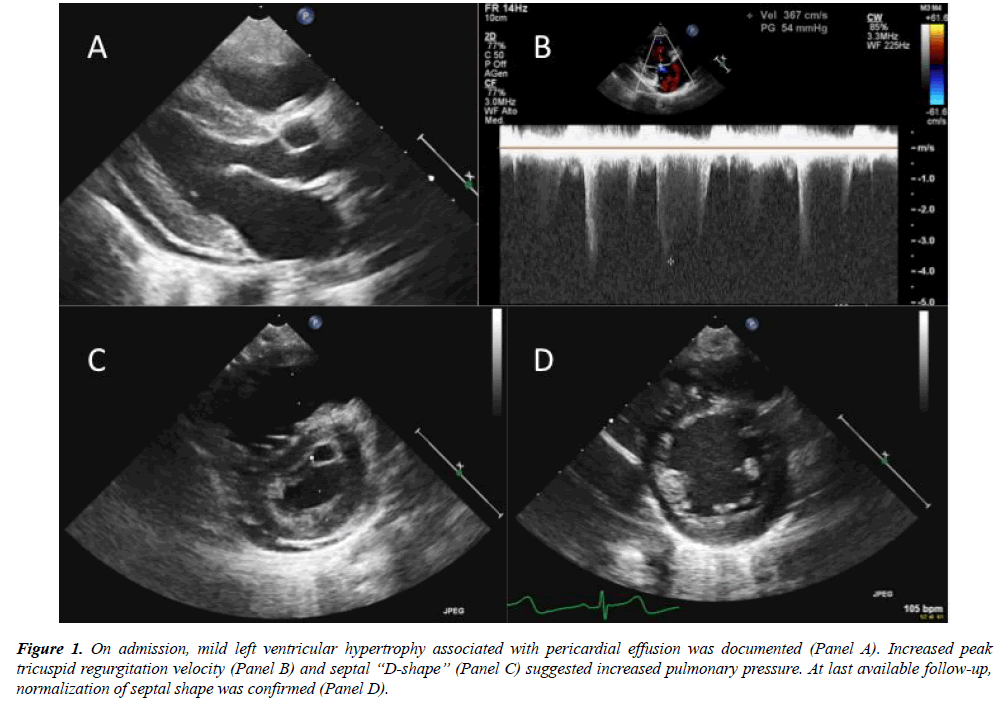
Given the negative history of fever and gastrointestinal symptoms, investigations for atypical HUS were considered. Extended metabolic screening revealed increased levels of plasmatic Homocysteine, Propyonilcarnitine (C3), blood and urinary Metylmalonic acid. Folic acid and vitamin B12 value resulted within normal range (Table 1). Peripheral blood smear highlighted hypersegmented neutrophils and schistocytes; the results lead to the diagnosis of methylmalonic acidemia and homocystinuria due to Cobalamin C deficiency (MMACblC), further confirmed at genetic test. The child underwent metabolic treatment, with rapid clinical improvement. Cardiologic evaluation confirmed progressive normalization of right pressure overload, even after sildenafil discontinuation. Systemic blood pressure gradually normalized, and antihypertensive therapy was discontinued within 6 months. At last available follow-up, after 2 years from discharge, the child showed regular growth and neurologic development. Echocardiography confirmed normal wall thickness, right chamber dimension and interventricular septum shape (Figure 1); no tricuspid regurgitation could be documented.
Figure 1: On admission, mild left ventricular hypertrophy associated with pericardial effusion was documented (Panel A). Increased peak tricuspid regurgitation velocity (Panel B) and septal ?D-shape? (Panel C) suggested increased pulmonary pressure. At last available follow-up, normalization of septal shape was confirmed (Panel D).
Discussion
Pulmonary hypertension (PH) is a rare condition in paediatric population, defined as an increase in mean pulmonary arterial pressure (PAPm) ≥25 mmHg at rest as assessed by right heart catheterization. PH may be primitive (either idiopathic or due to genetic mutation) as well as associated with multiple conditions as thromboembolism, hypoxia, congenital or acquired left and right heart diseases, septal defects, valvular disorders, connective tissue disorders, infections (HIV, schistosomiasis, hydatidosis), endocrinopathies, hematologic conditions and drug/toxin exposure [1] (Table 2). According to recent ESC guidelines, persistent PAH in the newborn has been classified as a distinct condition, given its unique pathophysiology [1]. Concerning metabolic disorders, glycogen storage disease [2] Gaucher disease [3] and primary mitochondrial disorders [4,5] have been reported as possibly associated with PAH, even if the underlying mechanism remains unclear.
| 1) Pulmonary arterial hypertension | |
| Idiopathic | |
| Heritable | BMPR2 mutation Other mutations |
| Drugs and toxins induced | |
| Associated with | Connective tissue disease Human immunodeficiency virus (HIV) infection Portal hypertension Congenital heart disease Schistosomiasis |
| Pulmonary veno-occlusive disease and/or pulmonary capillary haemangiomatosis | |
| Idiopathic | |
| Heritable | EIF2AK4 mutation Other mutations |
| Drugs, toxins and radiation induced | |
| Associated with: | Connective tissue disease HIV infection |
| Persistent pulmonary hypertension of the newborn | |
| 2) Pulmonary hypertension due to left heart disease | |
| Left ventricular systolic/diastolic dysfunction | |
| Valvular disease | |
| Congenital or acquired left heart inflow/outflow tract obstruction and congenital cardiomyopathies | |
| Congenital /acquired pulmonary veins stenosis | |
| 3) Pulmonary hypertension due to lung diseases and/or hypoxia | |
| Chronic obstructive pulmonary disease | |
| Interstitial lung disease | |
| Other pulmonary diseases with mixed restrictive and obstructive pattern | |
| Sleep-disordered breathing | |
| Alveolar hypoventilation disorders | |
| Chronic exposure to high altitude | |
| Developmental lung diseases | |
| 4) Chronic thromboembolic pulmonary hypertension and other pulmonary artery obstructions | |
| Chronic thromboembolic pulmonary hypertension | |
| Other pulmonary artery obstructions | Angiosarcoma/ intravascular tumors Arteritis |
Tabel 2: Differential diagnosis of pulmonary artery hypertension (from Simonneau G, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-41.
Whether primitive or secondary to another disease, the prognosis of PAH is frequently unfavourable, with a 3-years mortality rate of 25% [6].
Cobalamin C deficiency is a rare inborn error of cobalamin metabolism, commonly presenting with neurological, ocular, hematologic, renal and gastrointestinal symptoms. Even rare, association between MMACblC and atypical HUS is well known [7]. Conversely, secondary PAH has been described only in few paediatric cases, exiting in death in at least half of them [8-13]. The pathogenesis of renal and pulmonary damage is presumably due to arteriolar and capillary thrombosis with consequent micro-vascular dysfunction distinctive of Thrombotic Microangiopathy (TMA) [12,13]. TMA is a pathological process of micro-vascular thrombosis, consumptive thrombocytopenia and haemolytic anaemia, leading to multi-organ failure. Multiple conditions may present with TMA; gastro-intestinal or systemic infections (Escherichia coli, Shigella dysenteriae, Streptococcus pneumonia) are the most common in children, mainly occurring with renal and cerebral dysfunction (classical Haemolytic-uremic syndrome). Other less common causes comprise genetic mutations (ADAMTS13 protease deficiency), pregnancy, malignant hypertension, autoimmune disorders, drug exposure and transplantation (renal, hematopoietic stem cell transplant) [14-16]. Irrespective of the triggering cause, final step is abnormal complement activation, endothelial damage and platelet activation.
In the reported case, the concomitant presence of renal failure, thrombocytopenia, haemolytic anaemia and pulmonary artery hypertension, along with the exclusion of common causes of classical HUS, suggested the diagnosis of underlying metabolic disorder. The early diagnosis allowed a prompt administration of specific treatment, leading to a long-term positive outcome.
Conclusion
We reported a dramatic improvement after the beginning of metabolic treatment in a patient presenting with MMACblC complicated with both PAH and aHUS.
Complete and stable resolution of PAH was confirmed after 24-months follow-up; this may be due to an early diagnosis with prompt treatment administration. We therefore suggest considering MMACblC in paediatric patients presenting with PAH, after other more common causes have been already excluded, since early administration of specific treatment could completely change the patient's outcome.
Conflict of Interest
Authors have no conflicts of interest to declare.
Funding
No financial support has been received for the research, authorship and/or publication of this article.
References
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119.
- Li HP, Xie WM, Huang X, et al. Pulmonary hypertension in glycogen storage disease type II. Chin Med J (Engl). 2018;131(11):1375-6.
- Weinreb NJ, Barbouth DS, Lee RE. Causes of death in 184 patients with type 1 Gaucher disease from the United States who were never treated with enzyme replacement therapy. Blood Cells Mol Dis. 2018;68:211-7.
- Catteruccia M, Verrigni D, Martinelli D, et al. Persistent Pulmonary Arterial Hypertension in The Newborn (PPHN): a frequent manifestation of TMEM70 defective patients. Mol Genet Metab. 2014;111(3):353-9.
- Sproule DM, Dyme J, Coku J, et al. Pulmonary artery hypertension in a child with MELAS due to a point mutation of the mitochondrial tRNA(Leu) gene (m.3243A>G). J Inherit Metab Dis. 2008;31(3):497-503.
- Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension. Circulation. 2012;125(1):113-122.
- Chen M, Zhuang J, Yang J, et al. Atypical hemolytic uremic syndrome induced by CblC subtype of methylmalonic academia: A case report and literature review. Medicine (Baltimore). 2017;96(43):e8284.
- Profitlich LE, Kirmse B, Wasserstein MP, et al. Resolution of cor pulmonale after medical management in a patient with cblC-type methylmalonic aciduria and homocystinuria: a case report. Cases Journal. 2009;2:8603.
- Profitlich LE, Kirmse B, Wasserstein MP, et al. High prevalence of structural heart disease in children with cblC-type methylmalonic aciduria and homocystinuria. Mol Genet Metab. 2009;98(4):344-8.
- Iodice FG, Di Chiara L, Boenzi S, et al. Cobalamin C defect presenting with isolated pulmonary hypertension. Pediatrics. 2013;132:e248.
- Gündüz M, Ekici F, Özaydin E, et al. Reversible pulmonary arterial hypertension in cobalamin-dependent cobalamin C disease due to a novel mutation in the MMACHC gene. Eur J Pediatr. 2014;173(12):1707-10.
- Kömhoff M, Roofthooft MT, Westra D, et al. Combined pulmonary hypertension and renal thrombotic microangiopathy in cobalamin C deficiency. Pediatrics. 2013;132:e540.
- Beck BB, van Spronsen F, Diepstra A, et al. Renal thrombotic microangiopathy in patients with cblC defect: review of an under-recognized entity. Pediatr Nephrol. 2017;32(5):733-41.
- Fox LC, Cohney SJ, Kausman JY, et al. Consensus opinion on diagnosis and management of thrombotic microangiopathy in Australia and New Zealand. Intern Med J. 2018;48(6):624-36.
- Clark WF. Thrombotic microangiopathy: current knowledge and outcomes with plasma exchange. Semin Dial. 2012;25(2):214-9.
- Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29(3):191-204.