Review Article - Biomedical Research (2018) Volume 29, Issue 17
A systemic approach on understanding the role of moisture in pharmaceutical product degradation and its prevention: challenges and perspectives
Sukanta Roy1, Sabahuddin Siddique2, Shramana Majumder1, Mohi Iqbal Mohammed Abdul3, Syed Ata Ur Rahman3*, Durdana Lateef4, Shubhasis Dan5 and Anirbandeep Bose5
1Acharya and BM Reddy college of Pharmacy, Bengaluru, India
2Patel College of Pharmacy, Madhyanchal Professional University, Bhopal, Madhya Pradesh, India
3College of Pharmacy, Taibah University, Al-Madinah Al-Munawwarah, Kingdom of Saudi Arabia
4College of Science, Taibah University, Al-Madinah Al-Munawwarah, Kingdom of Saudi Arabia
5TAAB Biostudy Services, Jadavpur, Kolkata, India
- *Corresponding Author:
- Syed Ata Ur Rahman
College of Pharmacy
Taibah University
Al-Madinah Al-Munawwarah
Kingdom of Saudi Arabia
Accepted date: September 17, 2018
DOI: 10.4066/biomedicalresearch.29-18-978
Visit for more related articles at Biomedical ResearchAbstract
The review article explores the various problems associated with hydrolysis which occurs during formulation and the various solutions of it. The moisture content either the drug or the excipients affect the formulation, by hydrolysis, thus it is important to find out ways to prevent it and thus protect the formulation and provide a greater stability under processing and storage condition. The common moisture interactions which occur are water-solid interactions, water-amorphous solid interactions, drug-excipient interactions and the change in the crystal habit of the solids. The science behind the hydrolysis is due to the moisture sensitive functional group of the ingredient, and the other freely moveable living groups. Amides, lactams, esters, lactones, chloride are the functional groups most susceptible to hydrolysis. The hydrolysis of excipients, including both polymeric and non-polymeric also show great impact on the stability of the drugs. The excipients used in the form of sweeteners, plasticizers, solvents, surfactants, wetting agents, emollients, antioxidants, lubricants, preservatives, and etc. have effects on the drug stability. As a result, several solutions are found to prevent unwanted hydrolysis in different dosage forms. The main parameters which are likely to solve this issue are pH, buffers, surfactants, non-aqueous solutions, suspensions, lyophilization, packaging and an adequate proportion of the desiccant use.
Keywords
Hydrolysis, Drug degradation, Stability, Amorphous
Introduction
Hygroscopicity is an ingrained property of any substance, which is shown due to the presence of polar groups in the structure, and it occurred by either adsorption or absorption of moisture from the environment. A material when undergoes absorption or adsorption of moisture from the surroundings, it tends to change its physical characteristics like its volume, boiling point, melting point and some more. This moisture absorption also affects the product and process parameters like sticking, picking, bridging etc. Moisture adsorption is accountable for maintaining the physical and chemical stability of solids like the excipients, polymers in case of release studies. This makes it important to determine the moisture adsorption kinetics that contains rate of moisture uptake, and the equilibrium moisture content (EMC) especially in case of those drugs which undergo hydrolysis due to their hygroscopic tendency. The expression of water absorption is done in two ways, by the specific Absorption of Quality and by the specific absorption of volume. These values of the moisture absorption kinetics will be helpful in providing data about the solid dosage forms, the excipients and there will be knowledge on controlling humidity during production of the drugs and its storage [1,2].
The solid dosage forms are highly influenced by the moisture penetration. The moisture adsorption by an individual solid drug product at a specific relative humidity and temperature influences its flow, compression and the hardness. The percentage relative humidity (RH (%)) which is the ratio between water vapor in air to the highest amount of water vapor that can be held by air at specific temperature, expressed as percentage and helps to quantify the atmospheric water vapor pressure. In comparison to RH, EMC in a hygroscopic material is defined as the amount of moisture present in a material, when no further loss or gain of moisture occurs and the property becomes constant. This EMC depends on the material in concern to relative humidity and temperature of the air with which it has contact. Thus, for overcoming hygroscopicity of substances the moisture absorption rate must be determined [1-3].
The paper discussed here significantly specifies all the different problems that arises with hydrolysis and also the solutions for each one of them. Some of the major problems include polymeric, non-polymeric excipients and hydrolytic conditions. The solution for each and every hydrolytic problem due to moisture has been extensively discussed. The main approach made by the paper is diagrammatically expressing all the relevant issues related to hydrolysis which is very common on the practical ground and explaining each one of them with appropriate solutions.
Effect of Moisture upon the API and Excipient
Moisture interacts with drugs and excipient by following mechanism [3].
Mechanisms of water-solid interactions
It is known that water can react with crystalline solids by three different ways which are (i) deliquescence, (ii) the crystal hydrate formation and (iii) the adsorption of water vapor into the solid-air interface [4]. There is a chance for capillary condensation to arise at low relative humidity in solids having micro void spaces. There are two processes, deliquescence and capillary condensation that have the property of dissolving water-soluble substances by the formation of condensed or bulk water. The water molecules invade into the crystal lattice mostly in a well-defined molecular position inside one single cell and give rise to crystal hydrates. The rate of this invasion of the water under certain conditions into the single cell crystal is determined by the nature of stoichiometry and the position of water molecules along with the strength of the interaction. The water molecules remain adsorbed on the surface of the solid as adsorbed monolayers, the initial layer being hydrogen bonded to the solid, with another 2-3 other monolayers forming at higher relative humidities. This adsorption is usually reversible, and it changes with the decrease in relative humidity or by the increase in temperatures.
Water and amorphous solids
In comparison to the crystalline form of any substance, the amorphous part takes up more water because it has got a shape that is distorted, hence making it possible for more water to invade. This property is completely in contrast to that of adsorption, where the water intake based on the available surface area of the substance whereas this property depends on the mass of the solid substance. This property of water being absorbed to the solid is ideal to our interest. It is observed that this water absorbed in the amorphous solid acts as a plasticizer and increases the void volume of the solid by decreasing the hydrogen bonding between adjacent molecules with a decrease in the glass transition temperature.
Molecular disorder in crystalline solids
The amorphous structures arise when the regular and repeating arrangements of the atoms and molecules are changed by the defects or imperfections. Several processes in the pharmaceutical development like mechanical grinding, lyophilization or rapid drying result in those defects or imperfections and creates the local regions of molecular disorder. These molecules are known as activated molecules. The molecules have a tendency of exhibiting great chemical reactivity and solubility as they are in areas of local disorder and hence they are called activated. This activation is created due to the molecular mobility and the contact with more reactive chemical groups. Within the solid range if there is no distant order that results in amorphous characteristics of the solid. It is also observed that the areas of greater local disorders have a tendency to uptake increased amounts of water than the surfaces of the crystalline regions of the solids.
Drug-excipient interactions
The use of excipients for the formulation of drug is inevitable and it is also quite known that drugs in solid state in the presence of other solid excipients have the tendency to undergo certain significant physical and chemical changes, out of which the most important ones are increased rate of chemical degradation, decreasing in the extent of crystallinity and in the formation of molecular complexes. Two prime ways exist in which water can react with the excipients and those are (a) by the mechanism of adsorption or absorption water from the product can get into the drug through the vapor phase. (b) by interaction between the drug and the excipient, the sorbed water located between its physical contact can involve in the properties of the drug. These interactions are only responsible for causing the chemical degradation or catalysing or stabilizing amorphous structures of drugs against recrystallization to decrease energy in solid forms.
Problem Associated with Moisture Content which Leads to Hydrolysis of Different Drug and Excipient
The chemistry involve in hydrolysis of any molecule is basically depend upon the chemistry of an electrophilic carbon atom (often which is a carbonyl carbon) associated with a freely moveable living group. The character of living group plays an important role in modulation of the reaction depending on the presence of acidic or basic catalysts, and the electrophilicity of the carbonyl group (or other center) to which living group is attached. A schematic presentation of hydrolysis has been shown (reaction 1), where OH- act as nucleophile and L as living group (with or without charge) [5,6].
Hydrolysis constant can be written as mathematical equation as
ka=(RCOOH) (HL)/(RCOL) (H2O) → (1)
Few functional groups like acetals, ketals, hemi-acetals, hemiketals, imines, alkyl halides, phosphate esters, and sulfate esters are prone to hydrolysis and their presence may lead to hydrolytic instability even in the absence of catalysts. The presence of hydrolytically reactive sites must be viewed in terms of interpreting experimental findings rather than predicting hydrolytic instability because a few factors are related to the chemical structure of a drug and its environment. The Table 1 shows the examples of the hydrolysable functional groups and examples of the drugs which contain the functional groups.
| Functional groups | Structure of the hydrolysis prone group | Drugs associated with the functional group |
|---|---|---|
| Amide | 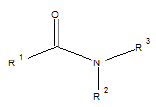 |
Acetaminophen, chloramphenicol, oxazepam, chlordiazepoxide |
| Lactam | 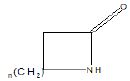 |
Penicillin G (b-lactam antibiotics) |
| Carbamic esters | 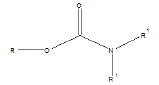 |
Loratadine, pipazetate |
| Ester | 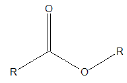 |
Atropine |
| Lactone | 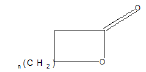 |
Warfarin |
| Imide | 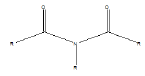 |
Barbiturates |
| Alkyl chloride | 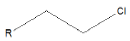 |
Chlorambucil |
| Acetal | 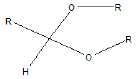 |
Erythromycin |
| Imine | 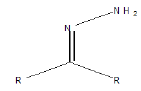 |
Diazepam, oxazepam, chlordiazepoxide |
Table 1. Following groups are prone to hydrolysis in most to least order.
Hydrolysis of Excipients
Alongside to drug hydrolysis, excipient hydrolysis also plays a significant role in drug stability. Often, the excipient is present in the formulation at high concentration so that even a small percentage of excipient degradation drives into compromising the purity of the drug product, affect its properties, or affect the drug product performance. The hydrolysis usually occurs
Non-polymeric excipients
• Hydrolysis of excipient can generate reactive that can affect the drug stability. The primary excipient functional groups like amides and esters that are susceptible to hydrolysis which yield carboxylic acids and either alcohols or amines. These groups have the potential to undergone reaction with either the drug or other excipients. The rate of relative reaction of excipient hydrolysis and byproduct reaction with drug, which can be consumed as quickly as it formed (when excipient hydrolysis is rate limiting). The drug reaction with the byproduct will follow pseudo first order reaction with respect to concentration of the excipient. Or else uptake of the reactive product is rate limiting and this intermediate will build up until the formation and uptake steps are equal. This will give the impression as a bi-model reaction rate for initial rapid hydrolytic drug degradation followed by slower terminal hydrolytic drug degradation rate. In aqueous media, alcohol and carboxylic acid product able to react with reactive drugs such as imines. In case of solids and non-aqueous solution, even the drugs which are less reactive can react with the products. The amine drugs having electrophile moiety can react even in aqueous solution. On other hand, the carboxylic acid or the ester group containing drugs trans-amination occurs from amide excipient to the drug.
• Excipient hydrolysis can modify the pH of a solution or affect the microenvironmental pH in a solid. When hydrolysis of an ester excipient occurs, the carboxylic acid product forms, which lowers the pH of its surroundings. When an ester used as a co-solvent (e.g. ethyl oleate or benzyl benzoate), its concentration can be high enough that even trace amounts of hydrolysis can cause significant pH shifts. Even though the buffers present to stabilize a solution pH, the pH may still shift significantly; i.e. the amount of acid formed can exceed the buffer capacity of the system.
• Excipient hydrolysis can alter the physical and pharmaceutical characteristics of the product. Although this effect is more prominentin polymeric excipient hydrolysis (see the “polymeric excipients” section), hydrolysis of some additives can affect process parameter like flow, disintegration, and dissolution rates, often by interaction with the API or other excipients.
• Flavors are usually associated with esters. Hydrolysis often will not only be lessening the pleasant odor and taste, but also impart an unpleasant odor and taste to the formulation.
• Table 2 shows the non-polymeric excipient and functional group present in them which are prone to hydrolysis by moisture.
| Non-polymeric excipients | Uses | Bond | Hydrolysable condition |
|---|---|---|---|
| Aspartame | Sweetener | Ester, amide | Hydrolyses with moisture; half-life, 20 d at pH 1, 7, 8; 250 d at pH 5 |
| Benzyl Benzoate | Solvent | Ester | If water is used as co solvent may hydrolyse |
| Chlorhexidine | Antimicrobial preservative; antiseptic | Imine | Hydrolyses in aqueous solution; 1.6% hydrolysis at pH 9, 30 min at 120°C. |
| Dibutyl sebacate | Plasticizer | Ester | Stable, not reactive with water and moisture. |
| Docusate sodium | Anionic surfactant; wetting agent | Ester | Stable in the solid state when stored at room temperature; |
| Gallates | Antioxidant | Ester | Rapid enzymatic hydrolysis. |
| Glyceryl monostearate | Emollient, emulsifying agent; solubilizing agent stabilizing agent; sustained-release agent; Tablet and capsule lubricant | Ester | Moderate hydrolysis under alkaline conditions |
| Isopropyl Myristate | Emollient; skin penetrant; penetration enhancer solvent | Ester | Resistant to hydrolysis |
| Lecithin | Emollient; emulsifying agent; solubilizing agent | Ester | Hydrolyses enzymatically |
| Palmitates | Emollient; oleaginous vehicle; solvent, antioxidant | Ester | Resistant to hydrolysis |
| Parabens | Antimicrobial preservative | Ester | Aqueous solutions at pH 3-6 autoclaved for 20 min at 120°C without decomposition and stable (10% decomposition) for 4 y sat room temperature; aqueous solutions at pH 8 or above undergo rapid hydrolysis (10% or more after 60 d at room temperature). |
Table 2. Non-polymeric excipient and their susceptibility towards hydrolysis in in different condition [7-12].
Polymeric Excipients
• It is observed that a huge variety of polymeric excipients are prone to hydrolysis. The derivatives of cellulose are more prone to hydrolysis. These derivatives which tend more to hydrolysis include hemi-acetals of cellulose, susceptible to hydrolysis.
• It is observed that a huge variety of polymeric excipients are prone to hydrolysis. The derivatives of cellulose are more prone to hydrolysis. These derivatives which tend more to hydrolysis include hemi-acetals of cellulose, susceptible to hydrolysis. Such derivatized cellulose includes cellulose acetate, hydroxypropyl methyl cellulose (HPMC) and cellulose acetate phthalate (CAP). This class of drugs undergo change in hydrolysis because their solubility and viscosity changes owing to the presence of increased amounts of free acid in the solution. This affects the in-vivo drug release performance.
• Drug release properties are changed due to the chain scissions and the strand modification occur due to hydrolysis in the presence of an acidic condition. These changes occur because of the composition of the cellulose backbone, which is made by condensation of sugars with hemi-acetal linkages. Along with the cellulosic derivatives, the non-cellulosic derivatives also hydrolyse, and the molecular weight gets reduced like in case of alginic acid, a linear glycuronan polymer, that is quite susceptible to hydrolytic strand scissions. Release rate is affected due to the hydrolysis in case of some controlled release, injectable dosage forms. In this case, polylactide-co-glycolide (PLGA) can be used as a copolymer. The in-vivo performance of the dosage forms changes gradually with the hydrolysis because the drug release profile in that case is directly dependent on the molecular weight of the polymer. Modifying agents like long chain polymers or natural gums, which are used in viscosity, based oral liquids or IM formulations. The viscosity is decreased by shortening the chain length with hydrolysis. Hydrolysis is not a problem as certain functional groups are present like the ester groups in polyacrylate and polymethacrylates that are very resistant to hydrolysis. Another reason for which the release rate is affected is due to the hydrolysis of the viscosity.
• Table 3 shows polymeric substances which are containing hydrolysable groups in different condition.
| Excipient | Uses | Bond | Reactivity | Hydrolysis effects |
|---|---|---|---|---|
| Cellulose acetate | Coatings, matrix for CR; Diluent; taste-mask | Ester linkages to acetyl groups | Slow hydrolysis at high temp/humidity | Acetic acid formation |
| Cellulose acetate phthalate (CAP) | Enteric coatings; matrix for CR | Ester linkages to acetyl and phthalyl groups | Slow hydrolysis at high temp/humidity. Slower release observed from coated tablets after 126 d | Phthalic, acetic acid formation; Changed enteric performance |
| Hydroxypropyl methyl cellulose phthalate (HPMCP) | Enteric coating matrix for CR; binder; microcapsule base | Ester linkages to phthalyl groups | Stable 3-4 y/RT, for 2-3 mos/40°C/75% RH, and as coating for 126 d/37°C. 8-9% hydrolyzed in 10 d/608°C/100% RH | Phthalic acid formation; changed enteric performance |
| Methylcellulose | Binding agent; disintegrant; matrix For CR; taste mask; sealant | Glucose-glucose acetal linkages | Hydrolyses pH<3 | Decreased MW; changed matrix performance |
| Hydroxyethyl cellulose | Thickening agent; binder; film coating agent | Glucose-glucose acetal linkages | Hydrolyses pH<5 | Decreased MW (loss of viscosity) |
| Hydroxypropyl cellulose | Binder, coating, matrix for CR; Thickening agent | Glucose-glucose acetal linkages | Hydrolyses at low pH | Decreased MW (loss of viscosity) |
| Alginic acid | Binder/disintegrant; Matrix for CR | Acetal linkages | Hydrolyses slowly at warm temperatures | Decreased MW (loss of viscosity) |
| PLGA | Controlled release matrix | Ester | Hydrolyses slowly | Loss of CR functionality |
Table 3. Polymeric excipients that potentially hydrolyse [7,13-21].
Methods to Overcome Hydrolysis Problem
In the section below, general solutions are advised for hydrolysis problems. Since each drug is a special case, the specific solutions (if any) that apply must be determined experimentally for the specific drug and formulation.
1) pH [20,21]: The pH of a substance is used to determine the shelf life of that substance. The change in pH can cause a lot of stability issues like robustness, despite using a good buffer system. Along with the stability issues with the formulations of the drug, another parameter that has an important role is the solubility of the drug and excipients at a pH with maximum stability. When a drug does not show optimum solubility, certain solubilizing techniques are adopted such as co solvents, surfactants or maybe complexation may be required. After the solubilization techniques are carried out, the pH is again checked for the resultant shelf life. The usage of surfactants, co-solvents and complexing agents used to dissolve a drug may also cause impact on the buffering capacity and the final pH of a formulation by changing the current pKa of the buffer or directly interacting with the buffering components. Thus, it becomes important to measure the final pH and effect on stability and shelf life. Alternative buffers might be used if adverse interaction occurs anyhow. In case of oral solution and suspension products, pH plays a major role in hydrolysis and excipients stability of the formulation (see the “nonpolymeric excipients” section), product taste, and the potential for precipitation of the drug in the stomach, resulting in bioavailability issues. To control the pH regulated hydrolysis the buffers generally used in the formulation are mentioned in Table 4.
| Buffer | pKa value | pH range of action |
|---|---|---|
| Phosphoric acid | 2.1, 7.2, 12.7 | 2-3.1, 6.2-8.2 |
| Tartaric acid | 3.0, 4.3 | 2.0-5.3 |
| Glycine | 2.34, 9.8 | 1.5-3.5, 8.8-10.8 |
| Glutamic acid | 2.1, 4.3, | 9.7 2-5.3 |
| Malic acid | 3.4, 5.1 | 2.4-6.1 |
| Benzoic acid | 4.2 | 3.2-5.2 |
| Ascorbic acid | 4.2, 11.6 | 3.2-5.2 |
| Succinic acid | 4.2, 5.6 | 3.2-6.6 |
| Triethanolamine | 8.0 | 7-9 |
| Diethanolamine | 9.0 | 8.0-10.0 |
| Adipic acid | 4.41, 5.28 | 3.4-6.3 |
Table 4. Buffer used in pharmaceutical formulation [22,23].
2) By formation of suspensions [24]: Suspensions are a way of increasing the stability when the drugs in the liquid formulations are hydrolytically labile. The concentration of the drug gets minimized and reduces the entire drug solubility as the reaction rates in less mobile solid states results in slower magnitudes. If the hydrolysis rate is neglected, the rate of degradation in suspension is determined by the rate of it dissolving capability of the drug at saturation. This is resulted by a zero-order reaction rate which is in most cases lower than the rate of higher concentrations in solution.
3) By using non-aqueous solutions [4,25]: This is another method to stabilize the active drug in a solution. The water moiety is replaced with the protic, aprotic or some other organic solvent. Organic solvents help to decrease the reaction rate by lowering the concentration of water. Water-causing hydrolysis is a major concern always in some solvents therefore solvolysis by alcohols and amines have replaced hydrolysis in most formulations.
4) By surfactants [26,27]: It is observed that to stabilize a drug, it is important to protect a drug from catalytic acid and base, which is done by surfactants-based micelles or liposomes mostly in case of hydrophobic drugs. Along with the charged micelles and liposomes even the non-ionic have shown good effects too in this case. As the polarity of the drugs increases, the drugs are less deeply enclosed in the hydrophobic core of the micelles.
5) Lyophilization [4]: This is also the art of removing the water and reducing mobility of the water, which results in improving the drug stability by using more complex manufacturing. This is a great method to stabilize a formulation from hydrolysis.
6) As a solid dosage forms [28]: There are many ways to stabilize hydrolysis in case of solids too, like choosing the right excipient. The correct form of excipient having lesser content of water in them will surely prevent unwanted hydrolysis. The preferred choice of excipients are the brittle ones, as lesser flow would be required during their processing, would also reduce molecular interactions and make them crystalline. The pH of the excipients plays another important role, as hydrolysis is catalysed under both acidic and basic media. There always remain slight changes in the micro environmental pH of the solution to reduce the stability of the dosage form. To stabilize the pH, appropriate buffers are used. Adding buffers in wet granulation becomes highly important. Crystalline drugs have chances of being for stable towards hydrolysis, hence salts, polymorphs or other substances with stronger lattice energies, i.e.; higher melting points. The processing of a drug into a formulation leads to a lot of changes and usually increases the hydrolysis. The force on tableting can be reduced to decrease the pressure on the active drug that can instead decrease hydrolysis. Particle sizes can be increased of the separation of two in different granulations can be done. These processing criteria’s help to decrease the hydrolysis.
7) By packaging [29-34]: Products which are prone to hydrolysis, packaging may provide a rational option for increasing the stability of the product. While using packaging, there are main two things to consider (i) the water vapor present in the head-space and within the dosage form itself and (ii) the permeation of water vapor through the container walls and cap. For both liquid and solid dosage forms. By packaging under controlled humidity conditions water vapor contribution from head space can be minimal. The permeation of water vapor into bottles or other packaging depends on the material. The rates of water vapor permeation through several commonly used packaging materials have been shown in Table 5. From below mentioned table it has been seen that, only glass and foil provide true barriers to water vapor permeation.
| Materials | Rate of water vapor permeation (g mm/(m2 d)) at 38°C and 90% RH |
|---|---|
| PVC | 1.8 |
| Polypropylene | 0.54 |
| HDPE | 0.12 |
| Aclar UltRx | 0.006 |
| Aclar 22A | 0.011 |
| Nylon | 6 7.5-7.9 |
| Oriented PET | 0.39-0.51 |
| Cold formed foil blister | <0.005 |
Table 5. Commonly used materials for packaging and their rate of water vapor permeation through them [29-34].
For solid dosage forms better moisture barrier characteristics at the same time allowing single dose use without exposing the bulk to moisture is blister packaging. Although this option is comparatively more expensive than bottle packaging, for some drugs this cost can be accepted. The water hindrance properties for several blister packaging options (typically multilayer coextrusions or laminates) have been studied under various temperatures and humidity conditions [23]. Characteristically, the greater the barrier properties, the higher the cost. Furthermore, many blister packaging materials that are decent for moisture, are poor for oxygen. Because of the greater surface area of a blister package per dose, moisture permeability will be a concern under challenging conditions with all the blisters except foil. Some blisters become noticeably more permeable at higher temperatures [23]. In terms of maintaining a reasonable relative humidity inside a blister under conditions of high moisture in the peripheral environment, only foil-foil blisters are likely to be adequate.
Desiccants
They contribute a major role in packaging for solving hydrolysis problems with solid dosages. Desiccants vary depending upon their rate of water adsorption capacity. They also vary in their ability to eliminate moisture as a function of relative humidity in the air. Following (Table 6) desiccants are used generally in the pharmaceutical formulation.
| Desiccant | RH reached (%) |
|---|---|
| Silica gel | 10-20 |
| Clay | 10-20 |
| Molecular sieves | 2-10 |
| CaSO4 | 15-30 |
| CaO | 1-25 |
Table 6. Desiccants and their RH capacity [35].
Conclusion
It is observed that the hydrolytic degradation of the drug mainly occurs due to unwanted moisture interference from API, excipients, during storage, processing or even while packaging. The effect of moisture on the drug occurs due to certain interactions, like the water-solid interactions, wateramorphous solid interaction, drug excipient interaction and molecular disorders of the crystalline structures. The hydrolysis of excipients occurs for both polymeric and nonpolymeric excipients. Non-polymeric excipients like aspartame, benzyl benzoate, chlorohexidine, docusate sodium are highly prone to hydrolysis and the polymeric ones like the hemi-acetals of cellulose and prone to hydrolysis [14-16]. The solution for them has been found for different pharmaceutical formulation including solid dosage forms. The modulation in pH, buffers, suspensions, non-aqueous solutions, surfactants and lyophilization are some of the solutions for unwanted hydrolysis. The pH determines the shelf life of a drug, so optimum pH levels are required to meet the expectations. Preparing suspensions instead of solutions is a way for hydrolytically labile substances. The use of organic solvents along with other protic or aprotic solvents is an alternative to the use of water or aqueous solvents. The micelles and liposomes that entrap water moiety into them can be used instead of the direct water. In short, the solution for hydrolysis is the correct choice of excipients, the pH of the formulation, the crystallinity of the drug substance, the processing conditions that includes the granulation techniques or other production parameters and most importantly the barriers in packaging [33-35]. The formulation development in unique in every case, depending on the type of drug or excipients used or maybe the method of production applied. This paper has overall tried to segregate and solve the various hydrolysis related problems.
References
- Hemant B, Saranjit S, Priyadeep M, Bhupindar S. Increase in the rate of moisture gain by hygroscopic drugs in the presence of non-hygroscopic water-soluble substances: study of the generalization of this hitherto unknown phenomenon, explanation to its occurrence and implications in formulation development. Asian J Pharm 2007; 1: 69-76.
- Arigo A, Jawahar N, Nikhitha S, Arun R. Effect of hygroscopicity on pharmaceutical ingredients, methods to determine and overcome: an overview. J Chem Pharm Res 2018; 10: 61-67.
- Claes A, George Z. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. Int J Pharm 1990; 67: 87-95.
- Kenneth CW, Roger C, Karen MA, Amy SA, Dan RA, Rebecca C. Hydrolysis in pharmaceutical formulation. Pharm Dev Tech 2002; 7: 113-146.
- Smith, MB, March MJ. Advanced organic chemistry. Reactions, mechanisms and structure (5th Edn.). Wiley New York 2001; 465-477: 1177-1178.
- Connors KA, Amidon GL. Stella VJ. Chemical stability of pharmaceuticals. A Handbook for Pharmacists (2nd Edn.). Wiley New York 1986; 32-62: 163-808.
- Kibbe A. Handbook of pharmaceutical excipients (3rd Edn.). American Pharmaceutical Association/Pharmaceutical Press London 2000.
- Yalkowsky SH, Davis E, Clark T. Stabilization of aspartame in water: organic solvent mixtures with different dielectric constants. J Pharm Sci 1991; 80: 674-676.
- El-Shattawy HE, Peck GE, Kildsig DO. Aspartame-direct compression excipients: preformulation stability screening using differential scanning calorimetry. Drug Dev Ind Pharm 1981; 7: 605-619.
- Patrunky M, Wollmann H. Stability testing of some drugs containing ester groups: benzyl benzoate, benzyl mandelate and propyl gallate. Part 11: stability of drugs and preparations containing the drugs. Zentbl Pharm Pharmakother Labdiagn 1982; 121: 851-856.
- Jaminet F, Delattre L, Delporte JP, Moes A. Effect of temperature of sterilization and of pH on stability of chlorhexidine in solution. Pharm Acta Helv 1970; 45: 60-63.
- Gui-You D, Satoh T. Pharmacokinetic studies on propyl gallate metabolism in rats. Res Commun Pharmacol Toxicol 1999; 4: 27-31.
- Santus, G, Baker RW. Osmotic Drug delivery: review of the patent literature. J Control Release 1995; 35: 1-21.
- Eastman Chemical Co. Technical literature: pharmaceutical ingredients-cellulosic enteric polymers 1994.
- Shin-Etsu Chemical Co. Ltd. Technical literature: hydroxypropyl methylcellulose phthalate 1993.
- Huikari A, Karlsson A. Viscosity stability of methylcellulose solutions at different pH and temperature. Acta Pharm Fenn 1989; 98: 231-238.
- Remunan-Lopez C, Bodmeier R. Mechanical water uptake and permeability properties of crosslinked chitosan glutate and alginate films. J Control Release 1997; 44: 215-225.
- Cohen S, Lobel E, Treygoda A, Peled Y. Novel in situ-forming opthalmic drug delivery system from alginates undergoing gelation in the eye. J Contro Release 1997; 44: 201-208.
- Baker RW. Controlled release of biologically active agents Wiley New York 1987.
- Martin A, Bustamante P, Chun AHC. Physical pharmacy (4th Edn.). Lea & Febiger Philadelphia 1993; 284-323.
- Sweetana S, Akers MJ. Solubility principles and practices for parenteral drug dosage form development. PDA J Pharm Sci Technol 1996; 50: 330-342.
- Gatlin LA, Gatlin CA. Formulation and administration techniques to minimize injection pain and tissue damage associated with parenteral products. Injectable Drug Development (1st Edn.). Interpharm Press Denver 1999; 401-422.
- Flynn GL. Buffers-pH control within pharmaceutical systems. J Parenter Drug Assoc 1980; 34: 139-162.
- Fife TH, Przystas TJ. Metal ion catalysis of anhydride hydrolysis. metal ion promoted water and hydroxide ion catalyzed reactions of mixed cinnamic acid anhydrides. J Am Chem Soc 1983; 105: 1638-1642.
- Parker AJ. Protic-dipolar aprotic solvent effects on rates of bimolecular reactions. Chem Rev 1969; 69: 1-29.
- Matos C, Chaimovich H, Lima JL, Cuccovia IM, Reis S. Effect of liposomes on the rate of alkaline hydrolysis of indomethacin and acemetacin. J Pharm Sci 2001; 90: 298-309.
- Mitchell AG. The hydrolysis of propyl benzoate in aqueous solutions of surface-active agents. J Pharm Pharmacol 1964; 16: 43-48.
- Carstensen JT. Solid state stability; incompatibility prevention techniques. Drug Stability New York 2000; 171-172.
- Allinson JG, Dansereau RJ, Sakr A. The effects of packaging on the stability of a moisture sensitive compound. Int J Pharm 2001; 221: 49-56.
- Pilchik R. Pharmaceutical blister packaging, Part 1, rationale and materials. Pharm Technol 2000; 68-77.
- Forcinio H. Choosing a blister material. Pharm Technol 2000; 26-30.
- Taborsky CJ, Foster MG, Lockhart H, Polgar B. Permeation of unit-dose blister market containers under USP and ICH conditions. Pharm Technol 2000; 38-42.
- Gerlowski LE. Water transport through polymers: requirements and designs in food packaging. Polym Prepr (Am Chem Soc Div Polym Chem) 1989; 30.
- Germano A, Lorenzi E, Calvo B, Guerra F. Compounding of materials capable of being heat-soldered and drug stability. Boll Chim Farm 1974; 113: 513-531.
- Dobson RL. Protection of pharmaceutical and diagnostic products through desiccant technology. J Packag Technol 1987; 1: 127-131.