Research Article - Biomedical Research (2017) Volume 28, Issue 1
A study on the prevention and management of surgical site infection post spinal surgery
Bo-Yong Hu, Xi-Hong Lu, Jun-Jie Ye, Yue-Gui Wang*Department of Orthopedics, Guangzhou Eighth People’s Hospital, Guangzhou, PR China
- *Corresponding Author:
- Yue-Gui Wang
Department of Orthopedics
Guangzhou Eighth People’s Hospital
627 Dongfeng East Road, Yuexiu District
Guangzhou, Guangdong
P.R.China
Accepted date: May 17, 2016
Abstract
Surgical site infection (SSI) is one of the most common infections in the health care settings with high financial burden and mortality rate.112 patients with infection of SSI were identified. 40 patients without SSI served as control group. Diabetes status, History of smoking, alcohol, other therapy before surgery is the main factors for potential risk of acquiring SSI. The SSI was studied from the day patient underwent surgery. Out of 88 patients (47 male and 41 female) with mean, age 45 ± 2.5 years. 42 (47.7%) patients had SSI at early stage and 46 (52.3 %) at later stage with mean duration of the hospital stay 287 ± 57 days. Among 40 controls, group (24 male and 16 female) with mean age 51 ± 4.5 years. Only 18 (45%) developed SSI. Microbiological profile of the SSI also studied both in study and control group patients. 18 (20.5%) patients implants were removed. 20 (47.6%) patients received β- lactam drugs. 24 (52.2%) patients underwent debridement. 12 (26.1%) received suppression therapy with antibiotics. In control group patients 5 (27.8%) patients implants removed. 6 (54.5%) patients received antibiotic therapy with Amikacin and cefozolin. 5 (27.3%) patients underwent surgical debridement. Control of diabetic status and reducing hospital stay before and after surgery with higher antibiotic prophylaxis can lead to decreased SSI, which in turn will reduce the financial burden of the patients after surgery.
Keywords
Infection, Therapy, Health Care, Burden, Surgery.
Introduction
Surgical site infection (SSI) is one of the most common infections in the health care settings. The prevalence of SSI ranges from 0-17 % [1-3]. The SSI rate after spinal surgery is >10%. In spinal surgery the risk and complication post-surgery are high mainly due to long-term hospital stay, which sometimes leads to HAI, prolonged antibiotic therapy, sepsis etc. The cost for care post-surgery is very high especially when SSI is acquired and leads to increased mortality rate [4].
The risk factors for acquisition of SSI are mainly due to implants used during surgery. Other risk factors are smoking, addiction to alcohol, elderly age group people, history of diabetes mellitus etc [5-12]. Awareness about risk factors helps in modification in preoperative and postoperative procedures. Spinal surgeries carry increased risk of infection than in orthopaedic procedures [13]. The higher incidences of infections are mainly due to complicated procedures and prolonged operation time involved in the spinal surgery [14,15].
SSI is not only the commonest complication post-surgery but has 8-23% of complication which consumes money, time, resources and has considerable strain on the morbidity and mortality of the patients [16,17]. The host factors are also one of the factor which increases susceptibility to infection favoring the environment for the pathogen to multiply such has the wards air in the theatre, Instruments used for surgery, Antibiotics all these has influence on SSI [18,19].
The orthopaedic implants used in bone and joint surgery especially in spinal surgery. The infections of the implants are mainly due to the adhesion of bacteria subsequently leading to biofilm formation [20,21].
The intra and extra operative environments are the main source for occurrence of SSI. Hence, management and prevention of SSI is important. By preventing the interventions patients overall outcome can be improved their by decreasing the time of hospital stay, increasing recovery time and reducing the cost of hospital stay.
Materials and Methods
This study was conducted in tertiary care Chinese hospital, china between 2014 Feb- 2015 October. The inclusion criteria for the patients in study group were patients under went spinal surgery with implants, use of instrumentation procedures were included. Patients without instrumentation procedures were excluded from the study. Patients with no implants but underwent surgery was also excluded from the study. Our control group patients consists of patients who underwent surgery other than spinal surgery were included.
The demographic details of the patients were collected from hospital record. Infection control officers based on CDC and prevention/National nosocomial infection surveillance definition of SSI identified all SSI’s. Total of 112 patients with infection of SSI were identified in which 24 of the patients were excluded because they had previous history of SSI or undergoing treatment for SSI. 40 patients without SSI were selected for control group with same inclusion criteria has that of study group. The study was approved by institutional ethical board.
Factors causing SSI
Many factors contribute risk of acquiring SSI. Among which age is the main factors followed by BMI of the patients. Diabetes status, history of smoking, alcohol and other therapies before surgery are the main factors for potential risk of acquiring SSI. Other factors include the antiseptics used for disinfecting skin, use of antibiotics before and after surgery, operating procedures etc. are the source for infection. The patients were given prophylactic antibiotics either in combination or alone with any cephalosporin with amino glycoside. The SSI was studied from the day patient underwent surgery and factors unavoidable in acquiring SSI and leading to the readmission/repeated surgery for SSI were monitored in our study.
Statistical analysis
Risk factors of SSI were analyzed statistically using 95% CI intervals. The significant level were analyzed with P>0.05 using Mann-Whitney U test. Multivariate logistics regression analysis was also done for finding the variables causing SSI. The statistical analysis was performed using SPSS software version 19.0.
Results
Out of 112 patients, 24 patients were excluded from the study and remaining 88 patients were assessed for the SSI. Out of 88 patients 47 (53.4%) were male and 41 (46.6%) were female patients. All the patients were in the age of 38-70 years with mean age 45 ± 2.5 years. Among 88 patients 42 (47.7%) patients had SSI at early stage. 46 (52.3 %) of the patients had acquired SSI at later stage during the hospital stay. Among the 88 patients, we were able to follow of only 76 patient’s far upto 1 year. The remaining 12 patients 4 (33.3%) expired and eight (66.7%) were not able to follow up due to other reasons. The mean duration of the follow up was 287 ± 57 days respectively. Among 40 control group patients only 18 (45 %) developed SSI during the study period. Of 40 control group patience 24 (60%) were male and 16 (40%) were female patients with mean age 51 ± 4.5 years (Table 1).
| Variables | Study group (n=88) |
Control Group (n=40) |
|---|---|---|
| Sex Male Female |
47 41 |
24 16 |
| Age (years) | 45±2.5 | 51±4.5 |
| Patients acquired infection at early stage |
42 | 11 |
| Patients acquired infection at late stage |
46 | 7 |
| No.of days of follow-up | 287±57days | 403±67 days |
| Diabetes Mellitus | 43 | 11 |
| BMI >40kg/m2 | 29 | 8 |
| Transfusion | 14 | 3 |
| Incontinence Pre-operative Post-operative |
12 11 |
1 4 |
| Previous history of SSI (during course of study) |
2 | 6 |
| Antibiotic therapy prior surgery | 26 | 10 |
Table 1: Study on various risk factors among study and control group population.
Microbiological investigation
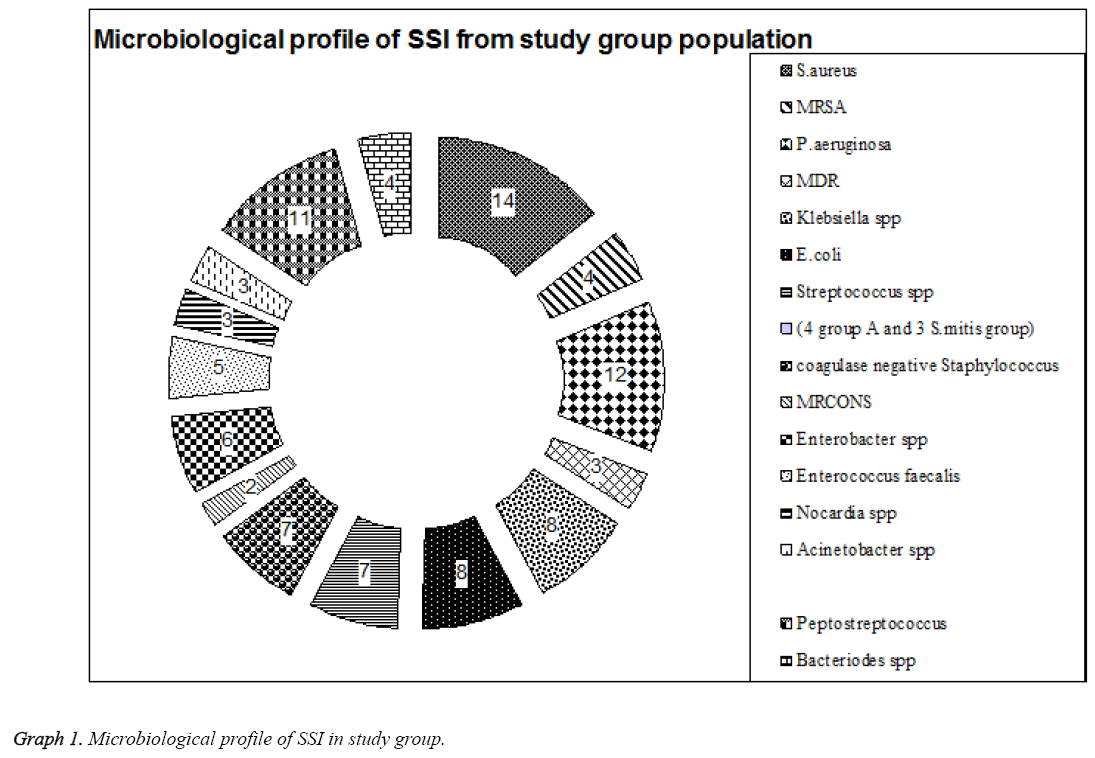
A Surgical wound swabs was taken in pairs and were sent to microbiological lab for Culture, they were inoculated on to Sheep blood agar, Chocolate agar and incubated aerobically at 37°C, and another swab was inoculated into Anaerobic medium and incubated in anaerobic Jar. In study, group population 88 patients had both early and late stage SSI with aerobic infection; the following are the list of organisms isolated. 14 (15.9%) were Staphylococcus aureus in which 4 (28.6%) were Methicillin resistant Staphylococcus aureus (MRSA). 12 (13.6%) were P. aeruginosa in which 3 (25%) were multi drug resistant strain (MDR), 8 (9.1%) were Klebsiella spp., 8 (9.1%) were E. coli, 6 (6.8%) were Enterobacter spp. 7(7.9%) were Streptococcus spp (4 group A and 3 S. mitis group). 7 (7.9%) were coagulase negative Staphylococcus (CONS) in which 2 (28.6%) were MRCONS. 5 (5.7%) were Enterococcus faecalis, 3 (3.4%) were Nocardia spp, 3 (3.4%) were Acinetobacter spp. Anaerobic infections was seen in 15 patients with 11 (12.5%) Peptostreptococcus and 4 (4.5%) Bacteriodes spp (Table 2 and Graph 1).
| S.no | Organism | Percentage of isolation |
|---|---|---|
| 1. | S. aureus MRSA |
14 (15.9%) 4 (28.6%) |
| 2. | P. Aeruginosa MDR |
12 (13.6%) 3 (25%) |
| 3. | Klebsiella spp | 8 (9.1%) |
| 4. | E.coli | 8 (9.1%) |
| 5. | Streptococcusspp (4 group A and 3 S.mitis group) |
7(7.9%) |
| 6. | coagulase negative Staphylococcus MRCONS |
7 (7.9%) 2 (28.6%) |
| 7. | Enterobacter spp | 6 (6.8%) |
| 8. | Enterococcus faecalis | 5 (5.7%) |
| 9. | Nocardia spp | 3 (3.4%) |
| 10. | Acinetobacter spp | 3 (3.4%) |
| 11. | Anaerobic Infections Peptostreptococcus Bacteriodes spp |
11 (12.5%) 4 (4.5%) |
Table 2: Microbiological profile of the patients with SSI in study group.
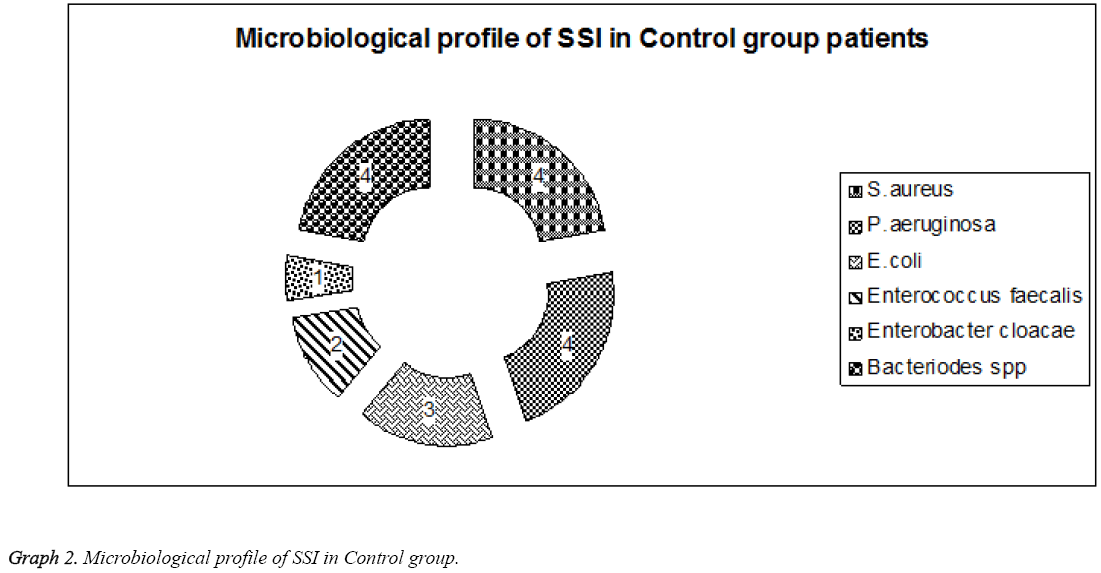
Most of the gram-negative bacilli were seen in patients with lumbar surgery whereas anaerobic infections were seen in patients with surgery below cervical regions. Gram positive infections were observed in patients with cervical surgery. This showed P>0.01 significance when compared between infection and region of surgery. In Control group with 18 cases of SSI 4 (22.2%) were S. aureus, 4 (22.2%) P. aeruginosa 3 (16.7%) were E. coli, 2 (11.1%) were Enterococcus faecalis, 1 (5.6%) Enterobacter cloacae. Four (22.2%) had anaerobic infections with Bacteriodes spp (Table 3 and Graph 2).
| S.no | Organism | Percentage of isolation |
| 1. | S. aureus | 4 (22.2%) |
| 2. | P. aeruginosa | 4 (22.2%) |
| 3. | E. coli | 3 (16.7%) |
| 4. | Enterococcus faecalis | 2 (11.1%) |
| 5. | Enterobacter cloacae | 1 (5.6%) |
| 6. | Anaerobic Infections Bacteriodes spp |
(22.2%) |
Table 3: Microbiological profile of the patients with SSI in Control group.
Both in study and control group patients the wound were seen in between organs space mostly or in the incisional areas and were implants were lodged. All the case of SSI documented pain with fever. The Median time of onset of SSI was 12 ± 35 days. 22 underwent repeat surgery either to remove implants with adhesion to clear the infection or to drain the pus from the infected site. When the risk factor smoking and diabetes were compared as a chance for acquiring SSI, it showed significance in univariate analysis. Transfusion showed no significant association with SSI with P>0.14.
Obesity and diabetes showed P<0.005 when smoking, diabetes, obesity were compared as a source of SSI with multivariate logistic regression showed positive value of significance.
Management of SSI with prophylaxis
Of the 42 (47.7%) patients with early acquisition of SSI 8 patients were removed with implants. 14 (33.3%), patients were started with oral prophylactic antibiotic course with cephalosporin (cefuroxime). 20 (47.6%) patients receive β- lactam drugs with moxifloxacin and sparfloxacin were also used for treating the patients. Of 46 (52.3%) patients with SSI at late stage 24 (52.2%) patients underwent debridement along with antibiotic therapy. 10 (21.7%) patients implants were removed and given β-lactam antibiotic therapy. 12 (26.1%) patients were given suppression therapy with antibiotics because their implants need to be retained for support. All the patients antibiotic therapy lasted until they were confirmed to be diagnosed as negative for SSI both by culture and by observing the healing of the wound (Table 4).
| S.no | Methods | Study group | Control group | ||
|---|---|---|---|---|---|
| Early acquisition (N=42) |
Late acquisition (N=46) |
Early acquisition (N=11) |
Late acquisition (N=7) |
||
| 1. | Debridement | - | 24 (52.2%) | 3 | 2 (28.6%) |
| 2. | Removal of Implants | 8 | 10 (21.7%) | 2 (18.2%) | 3(42.9%) |
| 3. | Retention of Implants | - | 12 (26.1%) | - | - |
| 4. | Antibiotic therapy Combination Alone |
20 (47.6%) 14 (33.3%) |
6 (54.5%) | 2(28.6%) | |
| 5. | Suppressive therapy | - | 12 (26.1%) | - | - |
Table 4: Methods used in our study for management of SSI.
Out of 40 control group patients 11 (27.5%) developed SSI at early stage. 2 (18.2%) patients implants were removed and 6 (54.5%) patients were started with antibiotic therapy with Amikacin and cefozolin for 3 months minimum. 3(27.3%) patients underwent surgical debridement and supported with antibiotics tobramycin and β-lactam drugs (Table 4). 7 (17.5%) patients had late acquisition of SSI in which 2 (28.6%) underwent debridement, 3 (42.9%) patients implants were removed due to adhesion of bacteria producing biofilm and 2 (28.6%) received combination therapy with tobramycin and β- lactam drugs (Table 4).
Discussion
In our study, we studied on various factors associated with risk of acquiring SSI post-surgery. Our study helps the health care professionals to plan and implement various measures for control and management of infections especially post-surgery. In our study the mean age of the patients in study group was 45 ± 2.5 years whereas in control group patients the mean age was 51 ± 4.5years which is concordant with the other studies which also show higher prevalence of SSI over age 48 years [22-24]. Age was one of the factors for SSI in our study [25-32]. Increased infection with increase in age is mainly due to decreased immunity [33]. When BMI of the patients was compared between study and control group patients. In study group patients 29 had >40 kg/m2 as BMI and in control group patients 8 had BMI >40 kg/m2 which is associated with risk of SSI [34-38].
The SSI was seen mostly inpatients who has prolonged hospital stay before surgery and after surgery has increased chance of acquiring infection along with colonization of resistant bacteria [39]. The patients who had implants placed in their spine had acquired more resistant infection due to adhesion of bacteria to implants leading to formation of biofilm on the surface [40]. Biofilm are the index of pathogenicity of the organisms.Biofilms are also known to correlate with the antimicrobial resistance (41). The most important factor contributing to virulence is Biofilm formation which is the initial step for colonization and adherence of the bacteria. Biofilms are the major concern in SSI infections which in turn leads to antibiotic resistance of the organisms complicating the treatment and increasing the duration of the hospital stay [42].
The post-operative incontinence seen in 11 patients in study population and 4 patients in control group patients showed contamination of the skin area [43] which is like previous studies which showed post-operative incontinence associated with risk of acquiring infection from skin area. Obesity also an important factor associated with SSI especially in spinal surgery many studies also had reported on the risk of SSI in spinal surgery [44,45]. Our study also had studied on the BMI factors as one of the main risk factors among SSI. In our study we have BMI >40 kg/m2 which is similar to other studies who had reported BMI of patients >35 to have higher risk of SSI [46].
To manage SSI in patients with higher BMI prophylactic antibiotics doses had to be increased. Other interventions such as contamination of the surgical site with faecal matters, Urine, and other skin flora since the patients are immovable most of the time after spinal surgery, which has to be emphasized especially in patients with increased chance of getting SSI.
The main reason for the lesser infection is mainly due to the use of prophylactic antibiotics before and after spinal surgery. However, older age, history of repeat spinal surgery or previous surgery is the main risk of SSI [44,45,47-49].
Limitation
In our study only small patients population was studied also, we focused more on spinal surgery than on other orthopedic surgeries. To analyze the potential risk larger population that included all the major surgeries has to be included to get a clear picture on the SSI management.
Our study highlights most of the potential risk factors of SSI in spinal surgery which most of the studies have not focused. We have also studied on the association of risk factors with multivariate analysis on which only few reports are available.
To Conclude, in our study we have found that patients with >BMI, diabetic, Obesity, postoperative incontinence had increased risk of acquiring SSI. This has to be monitored by introducing specific interventions such as diet control of the patients with nutritious food. Control of diabetic status and reducing hospital stay before and after surgery with higher antibiotic prophylaxis can lead to decreased SSI, which in turn will reduce the financial burden of the patients after surgery.
References
- Smith JS, Shaffrey CI, Sansur CA, Berven SH, Fu KMG, Broadstone PA. Rates of infection after spine surgery based on 108,419 procedures. Spine 2011;36:556-563.
- Willis-Owen CA, Konyves A, Martin DK. Factors affecting the incidence of infection in hip and knee replacement: an analysis of 5277 cases. J Bone Joint Surg Br 2010;92:1128-1133.
- Blam OG, Vaccaro AR, Vanichkachorn JS, Albert TJ, Hilibrand AS, Minnich JM. Risk factors for surgical site infection in the patient with spinal injury. Spine 2003;28:1475-1480.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 1999;20:725-730.
- Blam OG, Vaccaro AR, Vanichkachorn JS, Albert TJ, Hilibrand AS, Minnich JM. Risk factors for surgical site infection in the patient with spinal injury. Spine 2003; 28:1475-1480.
- Capen DA, Calderone RR, Green A. Perioperative risk factors for wound infections after lower back fusions. Orthop Clin North Am 1996; 27:83-86.
- Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine 2005; 30:1460-1465.
- Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg 1997; 86:975-980.
- Lim MR, Lee JY, Vaccaro AR. Surgical infections in the traumatized spine. Clin Orthop Relat Res 2006; 444:114-119.
- Olsen MA, Mayfield J, Lauryssen C, Polish LB, Jones M, Vest J. Risk factors for surgical site infection in spinal surgery. J Neurosurg 2003; 98:149-155.
- Olsen MA, Nepple JJ, Riew KD, Lenke LG, Bridwell KH, Mayfield J. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am 2008; 90:62-69.
- Picada R, Winter RB, Lonstein JE, Denis F, Pinto MR, Smith MD. Postoperative deep wound infection in adults after posterior lumbo-sacral spine fusion with instrumentation: incidence and management. J Spinal Disord 2000;13:42-45.
- Schimmel JJP, Horsting PP, de Kleuver M, Wonders G, andVan Limbeek J. Risk factors for deep surgical site infections after spinal fusion. European Spine Journal 2010; 19:1711-1719.
- Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR. Postoperative posterior spinal wound infections. Clinical Orthopaedics and Related Research 1992; 284: 99-108.
- Olsen MA, Mayfield J, Lauryssen C. Risk factors for surgical site infection in spinal surgery. Journal of Neurosurgery 2003;98:149-155.
- Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med 1991;324:377-384.
- Thanni LO, Aigoro NO. Surgical site infection complicating internal fixation of fractures: Incidence and risk factors. J Natl Med Assoc 2004;96:1070-1072.
- Lawal OO, Adejuyigbe O, Oluwole SF. The predictive value of bacterial contamination at operation in post‑operative wound sepsis. Afr J Med Med Sci 1990;19:173-179.
- Elek SD, Conen PE. The virulence of Staphylococcus pyogenes for man; A study of the problems of wound infection. Br J Exp Pathol 1957;38:573-586.
- Chevalier J, Gremillard L. Ceramics for medical applications: A picture for the next 20 years. J Eur Ceram Soc 2009; 29:1245-1255.
- Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release 2008; 130:202-215.
- Greene LR. Guide to the elimination of orthopedic surgery surgical site infections: an executive summary of the Association for Professionals in Infection Control and Epidemiology elimination guide. Am J Infect Control. 2012;40:384-386.
- Weigelt JA, Lipsky BA, Tabak YP, Derby KG, Kim M, Gupta V. Surgical site infections: causative pathogens and associated outcomes. Am J Infect Control. 2010;38:112-120.
- Cremet L, Corvec S, Bemer P, Bret L, Lebrun C, Lesimple B. Orthopaedic-implant infections by Escherichia coli: molecular and phenotypic analysis of the causative strains. J Infect. 2012;64:169-175.
- Oguntibeju OO, Nwobu RA. Occurrence of Pseudomonas aeruginosa in post-operative wound infection. Pak J Med Sci 2004;20:187-191.
- Cruse PJ, Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North Am 1980;60:27-40.
- Moylan JA, Kennedy BV. The importance of gown and drape barriers in the prevention of wound infection. Surg Gynecol Obstet 1980;151:465-470.
- Davidson AE, Clark C, Smith G. Postoperative wound infection: A computer analysis. Br J Surg 1971;58:333-337.
- Wukich DK, Lowery NJ, McMillen RL, Frykberg RG. Postoperative infection rates in foot and ankle surgery: A comparison of patients with and without diabetes mellitus. J Bone Joint Surg Am 2010;92:287-295.
- Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of thesurgical site after arthroplasty of the hip. J Bone Joint Surg Br 2005;87:844-850.
- Fascia DT, Singanayagam A, Keating JF. Methicillin-resistant Staphylococcus aureus in orthopaedic trauma: Identification of risk factors as a strategy for control of infection. J Bone Joint Surg Br 2009;91:249-252.
- Lucet JC, Chevret S, Durand-Zaleski I, Chastang C, Régnier B, Multicenter Study Group. Prevalence and risk factors for carriage of methicillinresistant Staphylococcus aureus at admission to the intensive care unit: Results of a multicenter study. Arch Intern Med 2003;163:181-188.
- Chandra RK. Nutrition, immunity, and infection: Present knowledge and future directions. Lancet 1983;1:688-691.
- Kaye KS, Anderson DJ, Sloane R, Chen LF, Choi Y, Link K. The effect of surgical site infection on older operative patients. J Am Geriatr Soc 2009;57:46-54.
- Silva QC, Barbosa MH. Risk factors for surgical site infection in cardiac surgery. Acta Paul En ferm 2012; 25:89-95.
- Chen TY, Anderson DJ, Chopra T, Choi Y, Schmader KE, Kaye KS. Poor functional status is an independent predictor of surgical site infections due to methicillin-resistant Staphylococcus aureus in older adults. J Am Geriatr Soc. 2010;58:527-532.
- Henriksen NA, Meyhoff CS, Wetterslev J, Wille-Jorgensen P, Rasmussen LS, Jorgensen LN. Clinical relevance of surgical site infection as defined by the criteria of the Centers for Disease Control and Prevention. J Hosp Infect. 2010;75:173-177.
- Lee DH, Kim SY, Nam SY, Choi SH, Choi JW, Roh JL. Risk factors of surgical site infection in patients undergoing major oncological surgery for head and neck cancer. Oral Oncol. 2011;47:528-531.
- Hanseen AD, Osmon DR, Nelson CL. Prevention of deep peri-prosthetic joint infection. J Bone Joint Surg 1996;78:458-471.
- Beer KJ, Lombardi AV Jr, Mallory TH, Vaughn BK. The efficacy of suction drains after routine total joint arthroplasty. J Bone Joint Surg Am 1991;73:584-587.
- Moretro T, Hermansen L, Holck AL, Sidhu MS, Rudi K, Langsrud S. Biofilm formation and the presence of the intercellular adhesion locus ica among staphylococci from food and food processing environments. Appl Environ Microbiol. 2003; 69: 5648-5655.
- Senthilkumar S,Anitha C, Vignesh S, Shanmugapriya R, Cherian KM. Detection of biofilm formation and mecA gene from clinical isolates of Staphylococcus sp. Adv. Biomed. Pharma.2014;1: 21-26.
- Perry JW, Montgomerie JZ, Swank S. Wound infections following spinal fusion with posterior segmental spinal instrumentation. Clin Infect Dis 1997; 24:558-561.
- Andreshak TG, An HS, Hall J. Lumbar spine surgery in the obese patient. J Spinal Disord 1997; 10:376-379.
- Wimmer C, Gluch H, Franzreb M. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord 1998; 11:124-128.
- Zerr KJ, Furnary AP, Grunkemeier GL. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg 1997; 63:356-361.
- Klein JD, Hey LA, Yu CS. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine 1996; 22: 2676-2682.
- Tenney JH, Vlahov D, Salcman M. Wide variation in risk of wound infection following clean neurosurgery. Implications for perioperative antibiotic prophylaxis. J Neurosurg 1985; 62: 243-247.
- Pons VG, Denlinger SL, Guglielmo BJ.Ceftizoxime versus vancomycin and gentamicin in neurosurgical prophylaxis: a randomized, prospective blinded clinical study. Neurosurgery 1993; 33:416-423.