- Biomedical Research (2014) Volume 25, Issue 1
A study of the components of metabolic syndrome in young adults.
SMR Usha, Chandrika N* and H.V. Shetty, Reena RDepartment of Biochemistry, Rajarajeswari Medical College and Hospital, Kambipura, Mysore road, Bangalore , India.
- *Corresponding Author:
- Chandrika N
Department of Biochemistry
Rajarajeswari Medical College and Hospital
Kambipura, Kengeri Hobli, Mysore Road
Bangalore-560074, India
Accepted Date: September 24, 2013
Abstract
Obesity is the epidemic of the twenty first century. In India the incidence of obesity continues to increase and prevalence among adolescents varies between 10% and 30%.The prevalence of metabolic syndrome which is a group of atherosclerotic risk factors is rising at an alarming rate among young adults of urban India. Central obesity which represents the visceral adipose tissue depots is the most prevalent manifestation of metabolic syndrome. Adipose tissue , a dynamic endocrine organ secretes pro-inflammatory factors called adipokines which causes meta inflammation thereby contributing to insulin resistance. We have undertaken a pilot study with ninety healthy MBBS students aged between 17 and 22 years from our Institution. These ninety students were further divided into non-obese, group I (n=63 ) and obese , group II (n=27) based on their waist circumference. We estimated fasting blood sugar(FBS), post prandial blood sugar(PPBS),lipid profile and serum insulin in both the groups.Insulin resistance (IR) was calculated using the Homeostasis Model Assessment (HOMA) equation. In this study we found that 3.3% of the study population of ninety students and 11.1% of the obese group had metabolic syndrome. 70.4% of the obese group had insulin resistance(IR) suggesting a strong correlation between obesity and IR.The increasing prevalence of childhood and adolescent metabolic syndrome implies a future global health burden. So we emphasize the urgent need for focus on prevention and treatment of metabolic syndrome in this age group through intensive health education and awareness programmes.
Keywords
Obesity, Insulin resistance(IR), Waist circumference(WC), Metabolic syndrome(MetS), Visceral adiposity.
Introduction
Obesity is a vexing problem in developed as well as developing countries. In developing countries, since both undernutrition and overnutrition are seen simultaneously, the double burden makes the situation more difficult. In India the incidence of obesity continues to increase and is occurring at a younger age, the prevalence among adolescents varying between 10% and 30% [1]. Obesity is one of the most significant contributors of morbid conditions like metabolic syndrome. The prevalence of metabolic syndrome in obese adolescents has been reported to be between 18 % and 42 % depending on the country of origin, suggesting an ethnic based association between obesity and metabolic syndrome [2]. There is an overwhelming moral, medical and economic imperative for early identification of individuals with metabolic syndrome because it is associated with an approximate doubling of the cardiovascular risk and five fold increased risk for incident Type 2 Diabetes mellitus (T2DM) [3]. Abdominal obesity is a marker of dysfunctional adipose tissue and is of central importance in the clinical diagnosis of metabolic syndrome [4]. Adipose tissue is known to express and secrete a variety of factors known as adipokines including leptin, adiponectin, resistin as well as cytokines and chemokines such as tumor necrosis factor-α, and Interleukin-6. These adipokines and cytokines which are pro-inflammatory markers are the underlying risk factors for metabolic syndrome. Also the release of adipokines by adipose tissue infiltrated macrophages leads to chronic inflammatory state that could play a central role in the development of insulin resistance [5]. In this study we intended to investigate and identify the components of metabolic syndrome among young adults and also examine the relationship between obesity and insulin resistance.
Materials and Methods
The study was conducted in Rajarajeswari Medical College and Hospital, Kambipura, Bangalore, Karnataka, India, from March 2012 to January 2013. The subjects of our study were MBBS students aged between 17 and 22 years . A written informed consent was taken from all the students involved in this study. The ethical clearance was obtained from the Ethical Review Board of the Institution. Students diagnosed with juvenile diabetes, anemia and those with any acute or chronic illness were excluded from our study.
These ninety students were further divided into two groups, group I , which comprised of sixty three non obese students with waist circumference of <80 cm in females and < 90 cm in males and group II which included twenty seven students who were obese ,with waist circumference of ≥80 cm in females and ≥90 cm in males. Fasting blood samples were collected from each of these ninety students under full aseptic precautions for the estimation of Fasting blood sugar (FBS), serum insulin and lipid profile which included total cholesterol(TC), high density lipoprotein (HDL) and triglycerides(TGL) . A postprandial blood sample (two hours after breakfast) was obtained on the same day and post prandial blood sugar(PPBS) was estimated.
Blood glucose(FBS and PPBS), serum total Cholesterol. HDL and Triglycerides were estimated on fully automated analyzer from Transasia company, ERBA EM 360.
Blood glucose(FBS and PPBS) was estimated by Glucose oxidase-Peroxidase (GOD-POD)method. Total Cholesterol by Cholesterol oxidase-peroxidase method, HDL was estimated by direct method, Triglyceride was estimated by Glycerol 3-phosphate oxidase method. VLDL was calculated by the formula VLDL=TGL/5. LDL was calculated using Freidwald's equation.
Serum insulin was analyzed on Roche Cobas e411 fully automated instrument by Chemiluminescence Immuno assay.
We estimated hemoglobin(Hb) concentration in the students in order to rule out anemia.The concentration of Hb was estimated by cyanmeth-hemoglobin method using EDTA blood sample.
Insulin resistance(IR) was derived using the Homeostasis Model Assessment equation (HOMA = fasting serum insulin (μunits/ml) × fasting plasma glucose (mmol/l)/22.5) . Students who had a HOMA score of more than 2.5 were taken as individuals with insulin resistance [6]. The reference method for assessing insulin sensitivity is the euglycemic glucose clamp. However clamps are ill suited because of extensive requirement of cost, time, labor and technical expertise. Simple surrogate indices of insulin sensitivity like HOMA have been developed and validated and a good corelation has been found [7,8,9].
Blood pressure was measured using sphygmomanometer in sitting position after five minutes rest. Waist circumference was measured according to WHO guidelines using a stretch resistant tape after normal expiration, at the midpoint between the lowest rib and the iliac crest. The measurement was made with the tape held snugly,but not constricting,and at a level parallel to the floor [10].
Statistical analysis
Values were expressed as Mean ± SD. Statistical comparisons were carried out by student 't' test. Correlations were done by calculating Pearson's correlation. All statistical analysis was done at 5% level of significance using statistical software SAS 9.2 and SPSS 15.0 .
Results
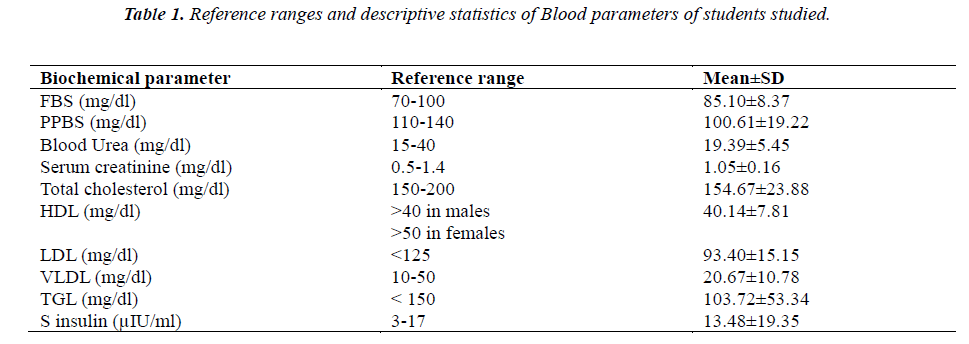
The reference ranges of the different biochemical parameters for the whole group (n=90) assessed in this study, along with mean and standard deviation is tabulated in Table 1.
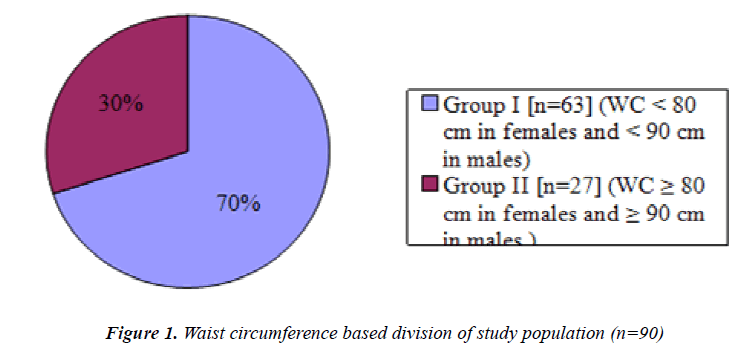
Figure 1 is a pie chart showing group I, non obese (n=63) students and group II, obese students(n=27) based on Waist circumference.
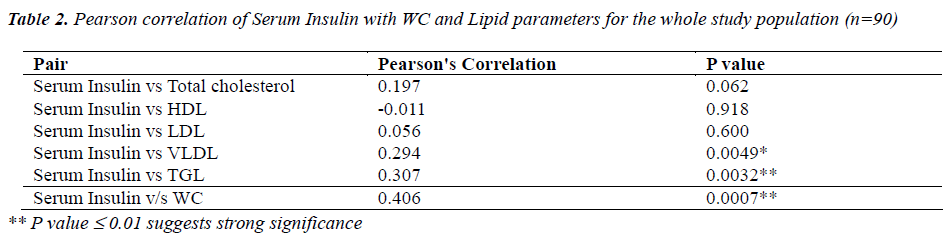
Table 2 shows correlation analysis of serum insulin levels with lipid parameters and WC for the whole group of ninety students . A positively significant correlation was observed between serum insulin and triglyceride levels( p<0.001) and also between serum insulin and WC (p<0.001).
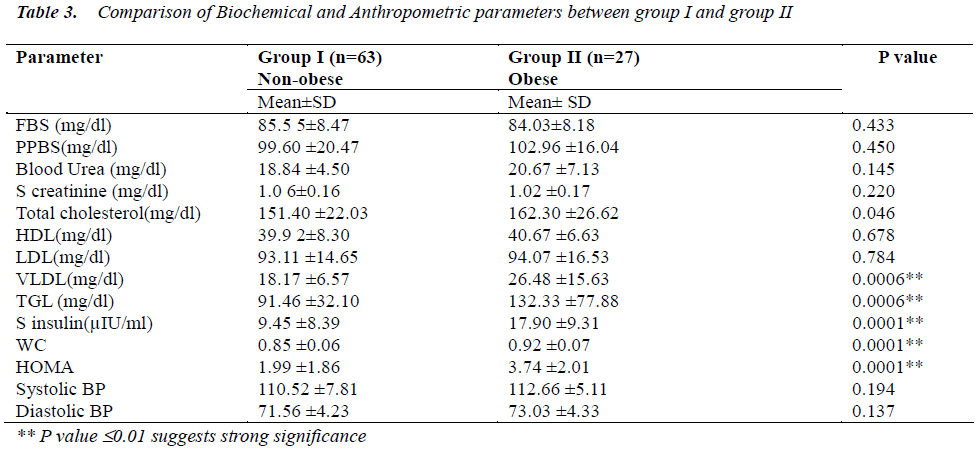
The important findings of this study is compiled in Table 3 which is a comparative table between biochemical and anthropometric measurements studied between non obese(group I) and obese(group II) . There were significantly higher levels of Total Cholesterol, VLDL, Triglycerides, serum insulin, and HOMA in obese as compared to non obese group.
Discussion
The new International Diabetic Federation (IDF) criteria defines metabolic syndrome(MetS) as a cluster of cardiovascular risk factors with the following components – increased waist circumference (WC ≥ 90 cm in males & ≥ 80 cm in females), elevated triglycerides (≥ 150 mg/ dl), low HDL (<40 mg/dl in males & <50 mg/dl in females), raised Blood Pressure ( systolic BP≥130 mm of Hg and diastolic BP ≥ 85 mm of Hg) and raised fasting plasma glucose( ≥ 100 mg/dl). Any three abnormalities out of five qualifies a person as having this syndrome. Central obesity is a prerequisite for metabolic syndrome and is often associated with insulin resistance [11].
In this study we have examined the components of metabolic syndrome among young adults in the age group of 17– 22 years. The most common MetS indicators observed were increased waist circumference, low HDL and increased triglycerides, while the least common components being, hyperglycemia and elevated BP. The percentage of students who had metabolic syndrome in the whole group of ninety students was 3.3%. Studies on larger population by Li et al [12], Singh R et al [13] and Tandon et al [14], have shown an overall MetS prevalence of 3.7%, 4.2% and 3% respectively among adolescents. Among the obese group in our study 11.1% had MetS, this is consistent with the studies of Lara Nasreddine [2] and Singh R [13], who have recorded a MetS prevalence of 19.2% and 36.6% respectively among obese group. 59 % had increased waist circumference and low HDL and 29 % had increased waist circumference and elevated triglycerides (accounting for two components of MetS), while 70.4 % of them showed insulin resistance.
Among the non obese group 40 % had low HDL, 20.6 % showed insulin resistance and 8 % had hyperinsulinemia. None of the students in this group had metabolic syndrome and also no subject in the entire study population had more than three components of MetS.
The emergence of obesity in adolescents and young adults can be contributed to significant nutritional shifts (consumption of non traditional fast food), lifestyle transitions and a steep increase in sedentary activities like television viewing and computer usage. These changes cause significant effects on body composition and metabolism, often resulting in increased body mass index (BMI) and visceral adiposity. Waist circumference has been recognised as the best indirect clinical index of visceral fat accumulation [15].
Obesity is often defined as a condition of abnormal or excessive fat accumulation in adipose tissue to the extent that health may be impaired. Until relatively recently, adipocytes were merely considered to be storage cells for fat. Now it is known that adipocytes are critical components of metabolic control and adipose tissue has emerged to be a dynamic endocrine organ that secretes a number of pro-inflammatory factors like cytokines and adipokines that are directly or indirectly involved in the pathogenesis of atherosclerosis, endothelial dysfunction, insulin resistance and vascular remodelling.This low grade inflammation is called meta-inflammation or metabolically triggered inflammation, an intermediate state between basal and inflammatory states [16].This leads to a cluster of conditions like dysglycemia, dyslipidemia, hypertension and pro-coagulant state known as metabolic syndrome, which is the most frightening complication of obesity. MetS is a clustering of components that reflects overnutrition, sedentary lifestyle and resultant excess adiposity. Visceral adiposity and insulin resistance appear to play a pivotal role in the development of MetS and its individual components [3].
The dyslipidemia associated with MetS is probably a consequence of increased transmembrane flux of fatty acids. Increased efflux of fatty acids from the adipocytes results in increased synthesis of triglycerides, cholesterol and Apo B particles by the liver. This results in elevated VLDL and LDL levels in the plasma. Ultimately cholesterol ester transport protein (CETP) mediated exchange of core lipids among the different lipoproteins results in small, dense LDL which are characteristic of the atherogenic dyslipidemia, thus setting a stage for future development of cardiovascular events [17].
The other dreaded complication of MetS is T2DM. Insulin resistance is believed to be a major final pathway from obesity to T2DM and it is defined as a decreased response of the peripheral tissues to insulin action. Endocrine, inflammatory and neuronal pathways link obesity to IR. The starting signal of inflammation is overfeeding and the pathway originates in all metabolic cells like adipocytes, hepatocytes and myocytes [18]. The pro-inflammatory cytokines like TNF-α and IL-6, secreted by the adipose tissue macrophages(ATMs) and the increased circulating free fatty acids associated with obesity lead to dysregulation of several cell intrinsic pathways that have negative effect on insulin signalling. They activate several serine/ threonine kinases (JNK, IKK, p38 MAPK) that inhibit insulin signalling either directly through insulin receptor substrate-1(IRS-1) or IRS-2 serine phosphorylation or indirectly through a series of transcriptional events mediated by NF-kB. The availability of increased fatty acids and adipokines in the liver can also be explained by the "Portal theory" [19].
29.6 % of obese students in our study were found to have normal insulin sensitivity. Studies of metabolic profile of obese individuals have shown similar findings. Such individuals are grouped as metabolically healthy obese individuals. The possible mechanism that could explain the protective metabolic profile in these group of people is that they have lower visceral, liver and muscle fat content than insulin resistant -obese people. The metabolically healthy but obese people have better ability to trap free fatty acids in their adipose tissue [20].
We have observed dyslipidemia and insulin resistance in a significant proportion of the non obese group in our study. Many hypotheses have been put forth to explain these findings.
The most common hypothesis is that Asian Indians may have a primary genetic susceptibility to develop MetS. Also Asian Indian men had higher insulin resistance than Caucasian men independently of generalized or truncal obesity indicating de novo insulin resistance [21].
Overall Asians and South Indians are classified as metabolically obese, that is, they have several metabolic derangements but are non obese by conventional standards. Asian Indians are reported to have larger adipocytes, which are more resistant to the action of insulin as compared to Caucasians [22].
It has also been hypothesised that South Asians have inherently lower fat storage capacity in their metabolically inert primary superficial subcutaneous adipose tissue compartments. (The vast majority of superficial subcutaneous adipose tissue is in the lower limbs). This results in the earlier utilization of metabolically active secondary compartments which are visceral and deep subcutaneous adipose tissue represented by abdomen and upper body respectively, for the storage of excess fat. This explains why at the same BMI, the atherogenic lipoprotein profile is more pronounced in South Asians than in Whites [17].
Conclusion
To conclude, obesity is an aggravating and contributing factor for the development of MetS at an early age. The conventionally non obese young Indians are also at risk of developing the metabolic complications at lower absolute masses of adipose tissue than White people.
Emergence of obesity and MetS in developing countries is due to a number of factors; the most important are demographic transition (shift to low fertility, higher life expectancy), nutrition and life style transition (consumption of non traditional fast food and sedentary life style), urbanisation and mechanisation. MetS presents a serious threat to the current and future health of the Indian youth.
Many studies and our findings suggest that MetS is far more common among adolescents than previously thought of. The importance of early screening for metabolic abnormalities in adolescents as well as young adults and the importance of early weight management intervention strategies should be emphasized and stressed, as the burden of co-morbidities may be reversed, if the health risks are identified and treated.
We recommend the screening of all young adults for the components of MetS. In addition the contributions of non-traditional risk factors (IR, pro-inflammatory state, endothelial dysfunction, small dense-LDL, genetic susceptibility) should also be considered while treating and defining MetS.
The major strength of our study is that the study group is a homogeneous population with respect to socioeconomic, cultural, educational and nutritional background.
The limitation of this study is that it is a cross sectional study with small sample size. Secondly, insulin resistance has been calculated by HOMA equation, rather than the accurate euglycemic clamp model.
Acknowledgements
We thank Dr.H.V.Shetty, Professor and Head of the Department, Dr.K.S.Priyadarshini, Professor, Dr. C.M. Bindu, Associate Professor, Dr.Deepti Gupta, Assistant Professor, Miss.Sowrabhi, Lecturer and Dr.Manjula, Post Graduate student, Department of Biochemistry, Rajarajeswari Medical College, Bangalore, India for their support and help during this research work. We also thank Dr.K.P.Suresh, Biostatistician, for helping us with the statistical analysis in this study.
References
- Kotian MS, Kumar GS, and Suphala S Kotian. Prevalence and Determinants of Overweight and Obesity Among Adolescent School Children of South Karnataka, India .Indian J Community Med. January 2010; 35(1): 176-178.
- Nasreddine L, Naja F, Tabet M, Habbal M-Z, Aida El-Aily A, Chrystel Haikal, Samira Sidani, Nada Adra1 & Nahla Hwalla.Obesity is associated with insulin resistance and components of the metabolic syndrome in Lebanese adolescents.Annals of Human Biology, March – April 2012; 39(2): 122–128.
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE , Wang H, and Eckel RH.The Metabolic Syndrome.Endocrine Reviews 2008; 29(7):777–822.
- Després JRm Lemieux I. Abdominal obesity and metabolic syndrome.Nature December 2006; 444, 881-887.
- Puente AB, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008; 34(1): 2-11.
- Matthews DR,Hosker JP, Rudenski AS,Naylor BA,Treacher DF, Turner RC.Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man.Diabetologia 1985; 28(7): s412-419.
- Katsuki A, Sumida Y ,Gabazza EC, Murashima S,Furuta M,Sasaki RA, Hori Y, Yano Y and Adachi Y. Homeostasis Model Assessment Is a Reliable Indicator of Insulin Resistance During Follow-up of Patients With Type 2 Diabetes Diabetes Care. February 2001; 24(2) : 362-365.
- Bonora E, Targher G, Alberiche M,Bonadonna RC, Saggiani F, Zenere MB,Monauniand T,Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity.Diabetes Care. January 2000 ;vol. 23 No. 1 :57-63.
- Lee HW, Muniyappa R, Yan X, Yue LQ, Linden EH,Chen H,Hansenand BC, Quon MJ. Comparison between Surrogate Indexes of Insulin Sensitivity/- Resistance and Hyperinsulinemic Euglycemic Glucose Clamps in Rhesus Monkeys.Endocrinology. February 2011; 152 ( 2) :414-423.
- Waist Circumference and Waist–Hip Ratio:Report of a WHO Expert Consultation Geneva, 8–11 December 2008.Pg 5-6.
- The IDF consensus worldwide definition of the metabolic syndrome.Available at http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf.
- Li Y, Yang X, Zhai F, Kok FJ, Zhao W, Piao J , Zhang J, Cui Z and Ma G.Prevalence of the metabolic syndrome in Chinese adolescents.British Journal of Nutrition 2008; 99:565–570.
- Singh R, Bhansali A, Sialy R, Aggarwal A.Prevalence of metabolic syndrome in adolescents from a north Indian population.Diabetic Medicine. February 2007;Volume 24, Issue 2:195–199.
- Tandon N, Garg MK, Singh Y, Marwaha RK. Prevalence of metabolic syndrome among urban Indian adolescents and its relation with insulin resistance (HOMA-IR). J Pediatr Endocrinol Metab. 2013; Vol 8:1-8.
- Santoro N., Amato A.,Grandone A.,Brienza C. Savarese P., Tartaglione N.,Marzuillo P.,Perrone L., Giudice ME. Predicting Metabolic Syndrome in Obese Children and Adolescents: Look, Measure and Ask. Obesity facts, Eu J of Obesity 2013; 6: 48-56.
- Shoelson SE, Lee J and Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006; 116(7): 1793 1801.
- Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N and Tchernof A.Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. International Journal of Epidemiology 2007; 36: 220-225.
- Emanuela F, Grazia M , Marco DR, Paola LM, Giorgio F, and Marco B. Inflammation as a Link between Obesity andMetabolic Syndrome.Journal of Nutrition and Metabolism. Volume 2012, Article ID 476380, 7 pages
- Qatanani M, Lazar MA .Mechanisms of obesity- associated insulin resistance: many choices on the menu. Genes Dev 2007; 21: 1443-1455.
- Karelis AD. Metabolically healthy but obese individuals. The Lancet. 2008; 372 (I9646): 1281-1283.
- Misra A,Vikram NK.Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications.Nutrition May 2004;Volume 20, Issue 5:Pages 482–491
- Misra A, Khurana L. Obesity and the Metabolic Syndrome in Developing Countries.The Journal of Clinical Endocrinology & Metabolism 2008;Vol. 93 no. 11 Supplement 1 :s9-s30.