- Biomedical Research (2010) Volume 21, Issue 3
A study of oxidative stress in cotton industry workers from Solapur city
A.N. Suryakar1*, R.V. Katkam2, V.N. Dhadke3 and R.B. Bhogade21Maharashtra University Health Sciences, Nashik, India.
2Department of Biochemistry, Dr. V. M. Govt. Medical College, Solapur, India.
3Department of Medicine, Dr. V. M. Govt. Medical College, Solapur, India.
- *Corresponding Author:
- A.N. Suryakar
Maharashtra University Health Sciences
Vani Road, Mhasrul, Nashik 422 004.
Maharashtra State, India
Accepted Date: March 27 2010
Abstract
The textile mill workers are continuously exposed to cotton dust which may lead to different respiratory disorders such as byssinosis, respiratory tract irritation, COPD etc. producing larger rate of decline in pulmonary functions and occupational hypertension. The present study was carried out to assess exposure effects of cotton dust on oxidant and antioxidant status which may induce related health hazards. 90 cotton industry workers and 30 healthy controls were screened for serum lipid peroxide (MDA) and serum nitric oxide as oxidants and superoxide dismutase, glutathione peroxidase, glutathione reductase and catalase as antioxidants. Total antioxidant status was also measured. The cotton industry workers were divided in three groups. Group I included workers with 5 to 9 years exposure to cotton dust while group II and III workers were exposed to cotton dust for 10 to 14 years and 15 to 19 years respectively. Highly significant increase in serum MDA and NO? was observed in all groups of cotton industry workers as compared to controls. The activities of SOD, glutathione peroxidase, glutathione reductase were found to be significantly decreased in group II and III workers. Our study demonstrated significant decrease in catalase activity and total antioxidant status in all groups of cotton industry workers. From our study, it is evident that cotton dust exposure induces oxidative stress among textile industry workers. As duration of exposure is increased, the effect is enhanced. This resulting oxidative stress may contribute to respiratory disorders observed in these workers.
Keywords
Cotton dust, oxidative stress, antioxidant.
Introduction
Due to extensive mechanization and use of chemicals, cotton industry workers are exposed to different occupational hazards such as accidents, fire, diseases due to exposure to cotton dust, disabilities because of exposure to high temperature, hearing impairment as a result of noise pollution, skin diseases caused by exposure to industrial chemical. Of all, the greatest health hazard is due to inhalation of cotton dust [1]. Constant inhalation of cotton dust causes various effects on respiratory system. The relation between exposure to cotton dust and respiratory disorders is well illuminated; they include mainly the byssinosis, respiratory tract irritation, chronic obstructive pulmonary disease (COPD) etc. producing larger rate of decline in pulmonary functions and occupational hypertension [1,2,3]. These disorders induced by inhalation of cotton dust seems at least partly, be mediated by free radicals or oxidative stress. These agents can affect epithelial, mesothelial and fibroblastic cells [4].
Free radicals are highly reactive species characterized by an unpaired electron in their outer orbital. Free radical reactions, including lipid peroxidation, are considered to be important factors in the pathogenesis of a variety of diseases. Free radicals can damage proteins, lipids, carbohydrates and nucleic acids. Plasma membranes are critical targets of free radical reactions. Oxygen derived free radicals can easily produce injuries to cell membranes by initiation of polyunsaturated fatty acid peroxidation. Malondialdehyde (MDA) is formed by fatty acids with two or more double bonds and is used as a measure of lipid peroxidation.
To defend themselves against these free radicals’ attack, cells have developed various antioxidant systems. Several enzymatic systems can detoxify free radicals like Superoxide Dismutase (SOD), catalase and a selenoprotein, Glutathione Peroxidase (GPx) [5]. In addition, some micronutrients can prevent the harmful effects of free radicals by nonenzymatic modes like vitamin E, vitamin C, glutathione etc. Under normal circumstances this defense system is able to cope up with free radicals in tissues by donating an electron to stabilize the free radicals, in doing so it can harmlessly decay itself or regenerate by another antioxidant. An imbalance between the production of reactive oxygen species (ROS) and the various antioxidant defenses can result in oxidative stress, which can arise from deficiencies of antioxidants and/or from increased formation of reactive oxygen species [6].
About 15% of total population in Solapur District is working in the cotton industry. Thus, considering the magnitude of textile cotton industries and large population employed in Solapur city situated in Maharashtra, an ideal group to study and as a public health problem, this study was undertaken. The purpose of the present study was to assess exposure effects of cotton dust on status of oxidants and antioxidants which may induce related health hazards and thereby to regard cotton dust as an ‘environmental toxin’.
Material and Methods
The present study was carried out in the Department of Biochemistry, Dr. V.M. Govt. Medical College, Solapur and SCSM General Hospital, Solapur. A total of 120 subjects were included in the study. Out of these 30 were healthy controls and 90 were workers employed in cotton industry.
Inclusion criteria
1. Healthy controls: Thirty healthy volunteers were selected from same locality and were matched for age, sex, dietary habits and socio-economic background. Only those who proved to be in a good state of health and free from any sign/s of chronic disease/s or disorder/s were included. None of them was exposed to any occupational dust.
2. Study group subjects: Workers employed in the cotton industry located in Solapur city were selected. Prior to the sample collection, subjects were interviewed for information of age, diet, tobacco habits, cotton dust exposure and medical history.
Exclusion criteria
The subjects having history of alcoholism, smoking, passive smoking and diseases which induce oxidative stress such as diabetes mellitus, respiratory diseases, cardiovascular diseases etc were excluded from study.
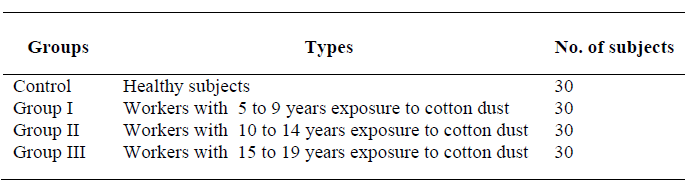
The study group subjects were further divided into three groups depending upon the exposure to cotton dust. The distribution of subjects in the present study was as follows:
The purpose of our study was explained to the subjects and their consent was taken. The study was approved by the institutional ethical committee. After obtaining prior consent, venous blood samples were collected from the subjects under aseptic condition. Out of 8 ml blood collected, 4ml was poured in sterile heparinized bulb and 4ml was allowed to clot. Serum and plasma were separated by centrifugation at 3000 rpm for 10 minutes at room temperature. All the samples were analyzed on the same day of collection. Serum malondialdehyde (MDA) levels were measured by reacting it with thiobarbituric acid at high temperature to form pink colored complex as in Kei Satoh method [7]. Nitric oxide was determined by Cortas and Wakid method [8], in which nitrate is reduced to nitrite by copper coated cadmium granules. This nitrite produced is determined by diazotization of sulfanilamide and coupling to naphthylethylenediamine to form purple complex. Assays for SOD, glutathione peroxidase, glutathione reductase, catalase and total antioxidant status were done by using kits by Randox Lab. Ltd. UK.
Statistical analysis
Statistical analysis was done by using student‘t’ test and the data were expressed as mean ± standard deviation. Probability values of P < 0.05 were considered to be statistically significant
Results
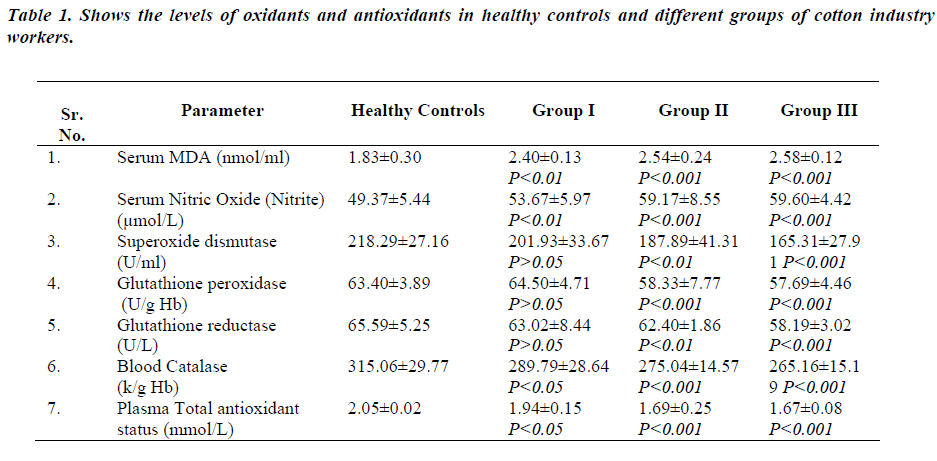
Table 1-shows levels of serum MDA, serum nitric oxide, superoxide dismutase activity, glutathione peroxidase, glutathione reductase, catalase and total antioxidant status in different groups of cotton industry workers and healthy subjects. Serum MDA and serum nitric oxide levels were significantly increased in all groups of cotton industry workers (group I-p<0.01, group II & IIIp< 0.001) as compared to healthy controls. There was slight but not significant decrease in superoxide dismutase and glutathione reductase activities of group I workers (p>0.05) as compared to healthy controls. But, Group II and III workers showed significantly reduced activities of superoxide dismutase and glutathione reductase (group IIp< 0.01, group III-p<0.001) as compared to healthy controls. There was no significant difference in glutathione peroxidase activity of group I workers and healthy controls (p>0.05). In Group II and III workers glutathione peroxidase activity was significantly reduced (p<0.001) as compared to healthy controls. Blood catalase activity and total antioxidant status was significantly decreased in all groups of cotton industry workers (group I-p<0.05, group II & III-p<0.001) as compared to healthy controls.
Discussion
Under normal conditions, there is a steady state of balance between the production of oxygen free radicals and their destruction by the cellular antioxidant systems. The oxygen free radicals, which accumulate via an imbalance between generation and scavenging, are believed to induce many disease states [9,10].
The indiscriminate proliferation of industrial organization without adequate safety measures in certain set up have resulted in various forms of stresses, one of them has been an oxidative stress created in the biological system due to cellular lipid peroxidation resulting in various disorders.
The present study revealed extensive increase in serum total lipid peroxide which was a resultant of concomitant increase in reactive oxygen species production in cotton industry workers.
When macrophages and neutrophils ingest microorganism or foreign dust, they undergo respiratory burst mediated by reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system. Consequently oxidants including superoxide anion, hydrogen peroxide, hydroxyl radical and hypoclorous radical are released. At low levels, these reactive oxygen species can increase chemokine, cytokine and adhesion molecule expression, thus amplifying the cascade of inflammatory mediators. At higher levels, these short lived molecules are implicated in a variety of tissue injury mechanisms.
Toxic oxygen metabolites released by activated neutrophils and macrophages synergistically increase toxicity of neutrophil elastases resulting in increased lipid peroxidation that alter membrane function (change in permeability). This lipid peroxidation inhibits production of prostacyclin and causes reorientation of arachidonic acid metabolism towards leukotriene, which stimulate inflammatory process in lung epithelium leading toward thrombosis, necrosis, airway obstruction, respiratory disorder etc [13,14].
Thus cotton dust inhalation cause lung inflammation and necrosis with the degree of oxidative damage. It is observed that occupational dust like cotton dust affects on large airways in early age and exposure period whereas involvement of small airways occurs as age and duration of exposure progresses.
Nitric oxide (NO˙) is a known bronchodilator and a potent inhibitor of platelet adhesion and aggregation [15]. NO˙ has significant effect on vascular smooth muscle tone and blood pressure [16]. In the present study we found significantly increased serum nitric oxide levels in all groups of cotton industry workers.
Inhalation of cotton dust induce overproduction of nitric oxide in many ways viz. increased expression of iNOS, cytokines, activated macrophages etc. whose deleterious effect lead to inflammatory lung disorders resulting in respiratory distress syndrome [17].
Increased levels of nitric oxide cause loss of local vascular regulation, vasoconstriction and mechanical blockage of vessels, all cause a reduction in pulmonary vascular region [11,13,14].
Macrophages and neutrophils which are capable of producing nitric oxide and superoxide are likely the host of a very powerful deleterious radical i.e. peroxynitrite (ONOO˙) anion which is relatively long lived reactive species. In this way NO˙ may magnify superoxide toxicity remarkably [18].
Present study depicted significantly lowered SOD activity in group I & II cotton industry workers. The decrease was substantial with inclination in the occupational dust exposure period in the study subjects as compared to nonexposed controls. SOD is a free radical metabolizing enzyme, catalyzing dismutation of superoxide radical to H2O2. This protects cell membrane from damage by highly reactive oxygen species [6]. The low activity of SOD in the study group could be due to inactivation of enzyme by cross linking or damage to DNA by lipid peroxidation induced by cotton dust exposure [19].
Statistically significant diminished levels of the glutathione peroxidase and glutathione reductase activities were seen in group II & III cotton industry workers when compared to healthy controls. Glutathione peroxidase removes both H2O2 and lipid peroxide by catalyzing the conversion of lipid hydroperoxides to hydroxyl acids in the presence of reduced glutathione [6].
Decrease in the activity of glutathione peroxidase in our investigation may be due to exhaustion of the enzyme by reactive oxygen species since oxidative damage to hemoglobin and cell membrane has been reported to reduce the activity of glutathione peroxidase [20]. The decrease in glutathione peroxidase and glutathione reductase activity may also be due to depletion of glutathione synthesis, decreased concentration of NADPH which in turn results in the loss of homeostasis of glutathione redox cycle or profound depletion of plasma protein sulphydryl groups following dust exposure [21].
Significant decrease in the activity of catalase was seen in the cotton industry workers when compared to those of control subjects. The depletion of antioxidant- catalase in cotton dust exposure population, is probably secondary to oxidative damage. Since exposure to pollutants is associated with free radical mediated tissue damage, the antioxidant should be in greater demand during dust exposure. This decrease in the catalase activity could also increase lipid peroxide due to overstress of singlet O2 and peroxyl radical which can crosslink with amino group of protein to form inter or intramolecular crosslinks thereby inactivating the enzyme [22].
Decreased levels of enzymatic and nonenzymatic antioxidants might be the cause of reduced ability of plasma to withstand ROS which is perceived as significantly diminished total antioxidant capacity among cotton industry workers.
Thus, it is evident from our study that cotton dust exposure induces oxidative stress among textile industry workers. As duration of exposure is increased, the effect was enhanced. Long term exposure to cotton dust results in macrophage/neutrophil migration into the airspaces which generate ROS production by opsonization, thereafter appear to precede the increased lung permeability which reflects a loss of epithelial tight junction integrity.
Acknowledgment
The study carried out was funded by Directorate of Medical Education & Research, Govt. of MAHARASHTRA, Mumbai, under the scheme of short term work as ‘Star research project’.
References
- Bonhuys A. Byssinosis in cotton weaving mill. Arch of Environ Health 1963; 6: 465-468.
- Thomas Pickring. Effects of occupational dust on blood pressure in man and women. Acta Physiologica Scandinavica 1997; 161: 125-128.
- Schnall PL, Landsbergis PA. Job strain and CVD. Am Rev Pub Health 1994; 15: 381-391.
- Halliwel B. Antioxidants in health and diseases. Ann Rev Nutr 1996; 16: 33-50.
- MacCall MR Frie B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radical Biol Med 1999; 26: 1034-1053.
- AALT Bast, Guibo, Haenen, Cecs JA. Oxidants and antioxidants state of art. Am J Med 1991; 91: 3S-10S.
- Satoh K. Serum lipid peroxide in cerebrovascular disorder determined by a new colorimetric method. Clin Chim Acta 1978; 90: 37-43.
- Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium reduction method. Clin Chem 1990; 36: 1440-1443.
- Halliwell B, Gutteridge JC. The definition and measurement of antioxidants in biological systems. Free Radic Biol Med 1995; 18: 125-126.
- Ryrefeldt A, Bannenberg G, Moldeus P. Free radicals and lung disease. British medical Bull 1993; 49: 588- 603.
- Henson PE, Johnston RB. Tissue injury in inflammation: oxidants proteinases and cationic proteins. J Clin Invest 1987; 79: 669-674.
- JM McCord, SM Stokes K Wong. Superoxide radical as a phagocyte produced chemical mediator of inflammation. In: G Weissmann, B Samuelsson and P Paoletti, Advances in inflammation research Vol 1,pp.-173-80(Raven press, 1979)
- Crystal RG. Oxidants and respiratory tract epithelial injury: pathogenesis and strategies for therapeutic intervention. Am J Med 1991; 91(3C): 39S-44S.
- RS Cotran, Vinay Kumar,Collins. Robbin?s Pathologic basis of disease, 6th Edition ,1999 by Hartcourt Asia Pvt. Ltd.,584, Orchard road, Singapure.
- Moncada S. Nitric oxide: physiology, pathology and pharmacology. Pharmacol Rev 1991; 43: 109.
- Czapski G. Role of reaction of nitric oxide with superoxide oxygen in biological systems: A kinetic approach. Free Radical Biol Med 1995; 19: 785
- Eiserich JP, Hiristova M, Cross CE, Jones AD, Freeman BA, Halliwel B. Formation of nitric oxidederived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998; 391: 393-397.
- Hogg N, Darley-Usmer VM, Wilson MT, Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J 1992; 281: 419-424.
- Crapo JD, Tierney DF. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol1974; 226: 1401-1407.
- Condell RA, Tappel AL. Evidence for suitability of glutathione peroxidase as a protective enzyme: studies of oxidative damage restoration and proteolysis. Arch Biochem Biophys 1983; 223: 407-416.
- Flohel J. Glutathion peroxidase fact and fiction. In: Oxygen free radical and tissue damage. Experta Medica 1979; 95: 122.
- Cheson BD, Churnutte JT, Babior BM. The oxidative killing mechanisms of the neutrophils. Prog Clin Immunol 1977; 3: 1-65.