Review Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2019) Volume 9, Issue 68
A Review of Ethnomedicinal, Phytochemical, Pharmacological and Toxicological Aspects of Eupatorium adenophorum Spreng
YC Tripathi1*, Nidhi Saini1, Nishat Anjum1, Praveen Kumar Verma21Chemistry and Bioprospecting Division, Forest Research Institute, PO New Forest, Dehradun 248006, India
2Systematic Botany Discipline, Forest Botany Division, Forest Research Institute, PO New Forest, Dehradun 248006, India
- *Corresponding Author:
- Y.C. Tripathi
Chemistry and Bioprospecting Division, Forest Research, Institute, PO New Forest, Dehradun 248006, India
E-mail: tripathiyc@gmail.com
Accepted on November 24, 2018
Abstract
Eupatorium adenophorum Spreng belonging to the family Asteraceae have traditionally been used as folklore medicine across the world. In traditional system of medicine, it is regarded as antiinflammatory, antimicrobial, antiseptic, analgesic, antipyretic, blood and coagulant. The present review summarizes the updated information concerning the ethnomedicinal, phytochemical, pharmacological and toxicological aspects of E. adenophorum. A thorough bibliographic investigation was carried out by analyzing worldwide accepted scientific data base (Pub Med, Google Scholar, Scopus SciFinder, and Web of Science), thesis, recognized books and other accessible literature from 1980 to 2017. The phytochemical and pharmacological studies demonstrated that E. adenophorum possess a wide spectrum of pharmacological activities, such as anti-inflammatory, analgesic, antipyretic, antioxidant, antibacterial, antifungal, antitumor, antioxidant, antiseptic and cytotoxic activities which could be attributed to the presence of array of phytochemicals of various groups including terpenoids, phytosterols, alkaloids, flavonoids, phenolic acids, coumarins, phenylpropanoids, sesquiterpene lactones, polysaccharides, and essential oil. Modern phytochemical and pharmacological studies have led to the isolation and characterization of a number of bioactive compounds from different parts of the plant as well as validation of its traditional medicinal uses. However, certain known toxic effects of E. adenophorum demand a thorough study of long-term toxicity and other toxicological aspects. Furthermore, the relationship of molecular structures of compounds of E. adenophorum with its various pharmacological activities needs further study and confirmation.
Keywords
Eupatorium adenophorum, Traditional uses, Phytochemistry, Pharmacology, Toxicity.
Introduction
Medicinal plants are indispensable natural resource constituting one of the potential sources of bioactive chemical entities for drug development [1]. Traditional medicinal uses of plants offer valuable clue to such drug development. It is estimated that about 60% of the world population and 80% of the population of developing countries rely on traditional medicine for their primary health care needs [2]. Medicinal plants satisfy the need of millions of ethnic and indigenous people living in tribal and rural sector of India. According to the report of the Ministry of Environments and Forest and Climate Change (MOEF&CC), Government of India, tribal communities in India use over 10,000 wild plants for primary health care [3,4], In recent years, despite incredible development in allopathic medicine, majority of people throughout the globe are opting for herbal healthcare system owing to their efficacy, safety and lesser side effects; as such, herbal medicines are gaining polarity and acceptance than ever before [4]. Medicinal plants related ethno medicinal knowledge has been an important guiding principle for research in the area of new drug development [5]. Persistent research on plants involves a persistent search for new phytochemical molecules with specific pharmacological efficacy and that are non-toxic and efficacious in controlling human diseases. In this respect, various species of Eupatorium hold great potential for in-depth investigation with scientific confirmation of their therapeutic properties thus making them potential sources of safer and more effective treatments.
Eupatorium: The Genus
The genus Eupatorium belongs to the tribe Eupatorieae, the largest among the 13 tribes in the family Asteraceae comprising nearly 1200 species distributed in the tropical, subtropical and temperate regions of America, Europe, Africa and Asia [6]. Plants of the genus are mostly perennial herbs, but a few are annuals, and some of the tropical species are shrubby or treelike typically bearing large clusters of purplish, pink, blue, or white flowers. Many species of the genus are considered as ornamental; a number of species however, are used in traditional medicine as a remedy to various diseases of humans, animals and plants [7-10]. Considerable phytochemical studies have been done in Eupatorium spp., and altogether more than 300 chemical compounds have been reported from the genus including flavonoids, terpenoids, pyrrolizidine alkaloids, phenylpropanoids, quinonoids, phytosterols and sesquiterpene lactones, essential oils, and some others [11]. Studies have shown that Eupatorium and its active principles possess a wide range of pharmacological activities, such as cytotoxic, antifungal, insecticidal, antibacterial, anti-inflammatory, and antinociceptive activities [12]. Essential oils obtained from Eupatorium spp. have deterrent impact on insects and lethal effect at the larval stage in the vector mosquito A. aegypti. It has been used as feedstock for the production of petroleum substitutes [13]. Eupatorium essential oils have been used for aromatherapy in alternative and complementary medicine.
Eupatorium adenophorum
Eupatorium adenophorum Spreng (syn. Ageratina adenophora (Spreng) R.M. King & H. Rob.) commonly known as Crofton weed, Eupatory, Sticky snakeroot, White thoroughwort and Mexican devil is an erect perennial herbaceous shrub growing 1-2 m (3.3 or 6.6 ft.) tall but occasionally reaching up to 3 m high [14,15]. Many upright stems are emerged from the woody rootstock of the plant. When young, its branched stems are green, reddish or purplish coloured and densely covered in sticky hairs whereas they become slightly woody and turn brownish-green or brown in colour at maturity [14]. Its roots are yellowish in colour and when broken or damaged emit a distinct carrot-like smell [15]. The leaves are oppositely arranged along the stems and are borne on stalks (petioles) that are 6-10 cm (2.4-3.9 in) long by 3-6 cm (1.2-2.4 in) in width [14]. The broad leaf blades are trowel-shaped, diamond-shaped (rhomboid), or triangular with bluntly or sharply toothed margins and sharply pointed and mostly glabrous tips while their stalks are often glandular pubescent [14]. The small compound flowers are clustered at the end of branches and blossom in late spring and summer. The small creamy white flower-head (capitula) consist of several tiny flowers (tubular florets) surrounded by two rows of greenish bracts and arranged in clusters at the tips of the branches. The tiny tubular florets (35 mm long) are white and contain both male and female flower parts (they are bisexual) [14,15]. Seeds (12 mm long and 0.3-0.5 mm wide) are slender, reddish-brown or blackish-brown in colour, and slightly curved having 4-5 five slight ribs which run longitudinally. Their bodies are glabrous topped with a ring (pappus) of numerous whitish hairs (34 mm long), which are readily shed [16]. E. adenophorum grows as weed in many parts of the world. It is a weed of roadsides, railways, pastures, fence lines, disturbed sites, waste areas and riparian zones in subtropical and warmer temperate regions and also found usually in urban open spaces, open woodlands, forest margins and rainforest clearings [17,18].
Distribution
E. adenophorum is native to Mexico and Costa Rica, Central America but has naturalized in many other parts of the world as an introduced species such as Europe, Oceania and Asia [19]. Initially, it been has grown as an ornamental plant, but rapidly become invasive into farmland and bush land worldwide. It was first inadvertently introduced to Yunnan around 1940 from the China-Burma border and then spread towards East and North covering Sichuan, Guizhou, Guangxi and Tibet Provinces of China [20,21]. Its rapid spread is due in part to its allelopathic competition with other plant species [22]. It is also a weed in Australia, where it was introduced to Sydney in 1904. It has spread along the coastline of New South Wales and southern Queensland [23]. It has also spread in Hawaii and the mainland United States, where it is recognised as a weed in ten states of the South and Southwest. It is rated a Class 4 Noxious Weed under the NSW Noxious Weeds Act of 1993 [24]. It is known as an invasive species in many tropical and subtropical countries and is almost naturalised in southwestern USA, southern Europe, Australia, New Zealand, South Africa, Spain, India, Philippine, Malaysia, Singapore, Indonesia, Papua, New Guinea, Thailand, Burma, Vietnam, Nepal, Pakistan, China, Pacific Islands and the Canary Islands [25,26].
Ethanomedicinal Significance
Various species of Eupatorium have been used in the traditional system of medicine across the world. E. adenophorum is accredited for diverse medicinal properties and finds therapeutic applications in traditional medicines as antimicrobial, antiseptic, blood coagulant, analgesic, antipyretic and phenobarbitone induced sleep enhancer [22,27,28]. The whole plant leaves and shoots have traditionally been used as folklore medicines in different parts of the world. The leaf juice is used to stop bleeding of cut and wounds, forming clots. Root juice is prescribed to treat fever. Pure juice of the leaf is poured in the eye to treat insomnia. A decoction of the plant has been recommended to treat jaundice and ulcers [18,29]. Conventionally, decoction of leaves has been applied on cut wounds to abate bleeding and used against infection of gum and tooth ache [30]. A decoction of leaves is given to cure stomach-ache among the tribal people of Meghalaya and Nagaland states of India [31]. Local populace of Kurseong and Darjeeling hill region in the Eastern Himalayas, use leaves of the plant for remedial purposes against oral and skin sores. Traditional practitioners of Darjeeling Himalaya prescribe the young leaves and shoots of the plant against dysentery [32]. In Nainital of Kumaun region of Uttarakhand state in India, leaf juice is used in blood clotting. In Garhwal region of the state, leaf paste is applied on cuts and wounds and paste mixed with mustard oil is useful for ulcer. In Nepal, the leaf juice is used as an antiseptic to treat cuts and wounds [33]. In Nigerian traditional medicine, it is used to treat fever, diabetes, and inflammation [34]. In India, leaves of the plants are pharmacologically regarded as astringent, thermogenic and stimulant and used in folk medicine for its antimicrobial, antiseptic, blood coagulating, analgesic, and antipyretic properties [35].
Phytochemistry
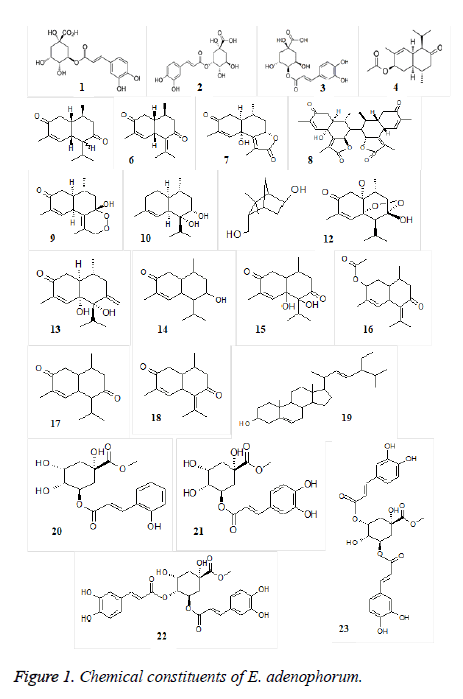
The genus Eupatorium in general and E. adenophorum in particular has been extensively investigated for its phytochemical constituents. Structurally diverse chemicals including (mono-, sesqui-, di-, and tri-) terpenoids, phenylpropanoids, polysaccharides, flavonoids, phenolic acids, coumarins, sterols and alkaloids have been reported from different parts of the plant [36-45], some of which were shown to possess allelopathic [46,47], phytotoxic [48] and antifeedant [42] activities. Sesquiterpenes including chlorogenic acid (5-O-caffeoylquinic acid, 5-CQA) (1) is one of the major bioactive compounds found abundantly in leaves of E. adenophorum. Neochlorogenic acid (3-O-caffeoylquinic acid, 3-CQA) (2) and cryptochlorogenic acid (4-O-caffeoylquinic acid, 4-CQA) (3) also found in leaves of the plant [45]; In addition, 2-deoxo-2-(acetyloxy)-9-oxoageraphorone (DAOA) (4), 9-oxo-agerophorone (OA) (5) and 9-oxo-10, 11-dehydroagerophorone (ODA, Euptox A) (6) are also found in leaves [49]. Xu et al. isolated compounds namely n-dotriacontane, β- sitosterol, stigmasterol, taraxasteryl palmitate, taraxasteryl acetate from the plant [50]. Ding et al. isolated a new sesquiterpenene lactone, eupqtoranolide and 11 known compounds from the flowers [51]. He et al. isolated four new cadinane sesquiterpenes, including a dimeric cadinane derivative and a peroxide cadinane analogue, from the leaves of E. adenophorum which characterized as (+)-(5R, 7S, 9R, 10S)-2-oxocadinan-3, 6 (11)-dien-12, 7-olide (7), (+)-7, 7′-bis ((5R, 7R, 9R, 10S)-2-oxocadinan-3, 6 (11)-dien-12, 7-olide) (8), (+)-(5R, 7S, 9R, 10S)-7-hydroxy-7, 12-epidioxycadinan-3, 6 (11)-dien-2-one (9) and (-)-(5R, 6R, 7S, 9R, 10S)-cadinan-3- ene-6, 7-diol (10) [38]. Zhao et al. isolated and characterized terpenoid constituents including a new monoterpene, (-)-(1R*, 2S*, 4R*, 5S*)-3, 3-dimethyl-5-hydroxybicyclo (2,2,1) hept-2- ylmethanol (11), two new cadinene sesquiterpenes namely, (-)- (5S*, 6S*, 7S*, 9R*, 10S*)-7-hydroxy-5, 7- epidioxycadinan-3-ene-2-one (12) and (+)-(5S*, 6R*, 9R*, 10S*)-5, 6-dihydroxycadinan-3-ene-2, 7-dione (13), and eight known terpene compounds [47]. Furthermore, cadinene derivatives such as 7-hydroxycadinan-3-ene-2-one (14) [52], 5,6-dihydroxycadinan-3-ene-2, 7-dione (15) [47], 2-acetylcadinan- 3, 6-diene-7-one (16) [22], cadinan-3-ene-2, 7-dione (17), cadinan-3, 6-diene-2, 7-dione (18) [53] and stigmasterol (19) [54] were isolated from hexane and ethylacetate concentrates of leaves and characterized spectroscopically [55]. A novel quinic acid derivative, 5-O-trans-ocoumaroylquinic acid methyl ester (20), together with chlorogenic acid methyl ester (21), macranthoin F (22) and macranthoin G (23) were isolated from the aerial parts of the plant [56]. Molecular structures of chemical compounds isolated and characterized from different parts of the E. adenophorum are depicted in Figure 1.
Essential oils of Eupatorium are richer in sesquiterpenes than in monoterpene compounds. Analysis of the light yellow coloured essential oil from aerial part of E. adenophorum showed the presence 45 components and the major compound was found to be torreyol (16.8%) 2-pentanone (7.71%), germacrene (7.49%), bornyl acetate (7.51%), 1-α-bisabolene (6.82%), δ-cadinene (6.4%), α-bisabolol (5.1%) [57]. However, remarkable difference has been recorded in the nature and relative content of different constituents of essential oils obtained from plants of different origins. Pala-Paul et al. reported major constituents of essential oil from the aerial parts as p-cymene (11.6%), α-phellandrene (5.7%), γ-curcumene (5.0%), δ-2-carene (5.0%), camphene (4.8%), and endo-bornyl acetate (4.4%) [58]. Essential oil from aerial parts of the plants from of northern India reported to contain amorph-4-en-7-ol (5.8%-17.7%), bornyl acetate (7.6%-15.9%), p-cymene (0.1%-16.6%), 3-acetoxyamorpha-4, 7 (11)-dien-8-one (0.3%-16.3%), α-phellandrene (1.5%-9.6%), camphene (0.1%-8.9%), α-bisabolol (1.7%-7.8%), α-cadinol (0.6%-6.2%), and amorph-4, 7 (11)-dien-8-one (3.2%-5.7%) [59]. Amorphenes (24.0%) including amorph-4-en-7-ol (9.6%), 3-acetoxyamorpha-4, 7 (11)-dien-8-one (7.8%) and amorph-4, 7 (11)-dien-8-one (5.7%) has been identified as the significant marker constituents of E. adenophorum along with p-cymene (16.6%), bornyl acetate (15.6%), and camphene (8.9%) [60]. Another sample of aerial parts essential oil from northern India found to contain 1-naphthalenol (17.5%), α-bisabolol (9.5%), bornyl acetate (8.98%), β-bisabolene (6.16%), germacrene-D (5.74%), α-phellandrene (3.85%) and a di-epi-α-cedrene (2.98%) [39].
Weyerstahl et al. reported the composition of the essential oil from flowers of E. adenophorum as α-phellandrene (15.3%), camphene (12.2%), bornyl acetate (10.6%), p-cymene (8.5%), γ-curcumene (4.5%) and 2-carene. Essential oil from inflorescences is dominated by sesquiterpenes (55.9%) with γ- cadinene (18.4%), γ-muurolene (11.7%), 3-acetoxyamorpha 4, 7 (11) diene-8-one (7.4%) and bornyl acetate (6.3%) as the major constituents [61]. The oil obtained from the roots contained both sesquiterpenes (34.3%) and monoterpenes (32.5%) in almost equal proportions with E-ecosmene (19.9%), γ-muurolene (10.1%), isothymol (7.5%), β-cadinene (7.0%) and α-phellandren-8-ol (5.9%) as the major constituents [35].
Pharmacology
E. adenophorum is traditionally known for its diverse medicinal properties and finds use in traditional medicines. There are many reports of using the whole plant, leaves and shoots of E. adenophorum as folklore medicines in different parts of the world. In Indian System of Medicine, leaves of the plants are pharmacologically regarded as astringent, antimicrobial, antiseptic, wound healer, analgesic, antipyretic, thermogenic and stimulant [62]. The aerial parts of the plants are claimed to be used as antimicrobial, antiseptic, blood coagulant, and analgesic [29]. Although several traditional uses of E. adenophorum are recognized, however, a scientific validity and supporting evidence is a pre-requisite for commercial exploitation. Some of the folklore medicinal claims have been scientifically validated for antiiflammatory [63], analgesic [64], antipyretic [65], antioxidant [66-68], antitumour [69], cytotoxic [70], antibacterial [71] antifungal, molluscicide potential and insecticidal, [72] activities. A brief description of different pharmacological activities investigated so far is presented hereunder.
Anti-Inflammatory Activity
Ethanolic leaf extract of E. adenophorum exerts antiinflammatory activity, likely through inhibition of IL1β, COX2 genes and quenching reactive oxygen species (ROS) such as hydroxyl radical. Intravenous administration of the leaf extract increased the number of CD4 T cells in spleen and tumour necrosis factor (TNF)-α, an established proinflammatory cytokine in serum of delayed DTH mice. The extract also induces TGFβ encoding a cytokine involved in tissue repair mechanism and inhibits expression of another proinflammatory cytokine gene IL1β and down-regulates cycloxygenase 2 (COX2) genes responsible for metabolism of inflammatory mediators like prostaglandins. Furthermore, anti-inflammatory role of ethanolic extract of leaves is also revealed through its property to inhibit hydroxyl radical generation. The ethanolic leaf extract of E. adenophorum has found to suppress efficiently the inflammatory reaction set in foot paw by injecting two different doses of 2, 4DNFB. The topical application of the extract found to be more effective in inhibition of foot paw swelling and gaining normalcy faster than its intravenous administration [63].
Analgesic Activity
The methanol extract of E. adenophorum leaves showed significant analgesic activity as compared to standard drugs diclofenac sodium and pentazocine in acetic acid-induced writhing test, tail immersion test and tail flick test models. The leaf extract significantly increased the required induction time to produce the writhing movements and demonstrated significant analgesic activity in tail flick and tail immersion tests respectively. Under acetic acid induced writhing reflex model of analgesic activity, the number of writhing movements were significantly less in the mice treated with the methanol extracts of leaves when compared to that of vehicle treated control group thereby suggesting it's both peripheral and central analgesic effect [64].
Antipyretic Activity
The anti-pyretic effect of methanol extract of E. adenophorum leaves on yeast induced pyrexia at the doses of 300 and 400 mg/kg body weight showed decreased yeast incited rise in body temperature of rats. The standard drug paracetamol significantly reduced the yeast-provoked elevation of body temperature at a dose of 150 mg/kg. The result suggested a significant antipyretic effect methanol extract of leaves in rats comparable to that of paracetamol (standard drug). The bioactive constituents like triterpenoids, β-sitosterol known to have antipyretic effect might be responsible for the antipyretic efficacy of the plant [65]. The aqueous extract of leaves reported to exhibit significant antipyretic activity in the dosage of 300, 400 and 500 mg/kg body weight as compared to standard drug paracetamol [73]. This validated the tradition medicinal claim on antipyretic efficacy of the plant [35].
Antioxidant Activity
The ability of ethanolic extract of E. adenophorum leaves in quenching the generation of hydroxyl radical has been tested and found effectual [63]. The quinic acid derivative including 5-O-trans-o-coumaroylquinic acid methyl ester, chlorogenic acid methyl ester, macranthoin F and macranthoin G isolated from the aerial parts of the plant were tested for their antioxidant activity against DPPH (1, 1-diphenyl-2- picrylhydrazyl) radical and found effective [56]. The essential oil and cadinenes from leaves of the plant were evaluated for antioxidant activities using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging protocol and the ferric reducing ability assay (FRAP) which showed antioxidant activity comparable with the standards i.e. ascorbic acid, tert-butyl-4- hydroxy toluene (BHT), and gallic acid, thus suggesting their potent antioxidant activities [66]. E. adenophorum oil further tested by DPPH and β-carotene bleaching methods showed potent antioxidant activity with IC50 values were 8.3 and 4.2 μl, respectively suggesting it to be a potent antioxidant agent [67]. Another study reported that oral administration of E. adenophorum leaf extract causing oxidative stress in mice by reducing the activities of antioxidant enzymes such superoxide dismutase (SOD), catalase (CAT) and reduced glutathione (GSH) and marked increase in lipid peroxidation (LPO as compared to control suggesting that its oral administration for medicinal purposes without proper dosing could be potentially toxic to higher animals [68].
Wound Healing Activity
Wound healing potential of E. adenophorum (A. adenophora) ethanolic extract formulated as gel was estimated by excision and incision wound models. Ethanolic extract gel showed highly significant activity as compared to pure gel control. The plant exhibited moderately significant wound healing potential in both excision as well as incision wounds as evident from the tensile strength, epithelialization time and wound index data. The plant showed strongly significant (p<0.01) wound healing potential in excision as 90.98% wound contraction and 36.16% reduction in epithelialization time. In incision model, significant increase (37.86%) in tensile strength was recorded as compared to pure gel control. Wound index data clearly showed that quality of healing was much better in plant extract treated animals as compared to pure gel control [74].
Antibacterial Activity
Leaves and stems extracts of E. adenophorum reported to exhibit antibacterial effect towards Proteus spp., Salmonella spp., Staphylococcus spp., Bacillus subtilis, B. thurengiensis, Enterobacter aerogenes, Salmonella paratyphi, Staphylococcus aureus, B. cereus, Proteus mirabilis; and water solvent extract showed antibacterial effect towards Peudomonas aeruginosa, E. coli, S. aureus, Staphylococcus spp., Citrobacter frundii, Proteus spp., Bacillus subtilis, Enterobacter aerogenes, Salmonella spp., Salmonella paratyphi, Bacillus thurengensis [75]. Essential oil from aerial parts exhibited antibacterial activity against Arthrobacter protophormiae, Escherichia coli, Micrococcus luteus, Rhodococcus rhodochrous, and Staphylococcus aureus [39]. Arvind et al. reported the antibacterial activity of petroleum ether extract of E. adenophorum leaves against Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, Escherichia coli, Klebsiella aerogenes and Pseudomonas aeruginosa [76]. In another study, the essential oil showed significant antibacterial activity against both gram positive (Klebsiella pneumoniae and Staphylococcus aureus) and gram negative (Escherichia coli and Proteus vulgaris) bacteria. However, gram-positive bacterial strains showed greater susceptibility than gram-negative bacteria suggesting that the essential oil is more active against gram- positive bacteria [57]. The bioactive compounds 5-O-trans-o- coumaroylquinic acid methyl ester, chlorogenic acid methyl ester, macranthoin F and macranthoin G isolated from the aerial parts of E. adenophorum showed in vitro antibacterial activity toward bacterial strains, Salmonella enterica with MIC values of 7.4 and 14.7 μM, respectively [56]. The inflorescence oil showed higher antibacterial activity against Klebsiella pneumoniae, while the root oil was more effective against Staphylococcus aureus [35].
Antifungal Activity
Extracts of E. adenophorum leaves and stems have been reported to inhibit fungal strains including Fusarium moniliformae, F. eroliferum, F. proliferatum and F. oxysporum [75]. Tian et al. found that the volatile oil extracted from E. adenophorum inhibited four types of fungal pathogens [77]. Petroleum ether extract of E. adenophorum leaves showed antifungal activity against Aspergilus niger, Aspergilus candidus and Candida albicans [76]. Compound 5-O-trans-o- coumaroylquinic acid methyl ester further found to display in vitro anti-fungal activity against spore germination of Magnaporthe grisea with an IC50 value 542.3 μM [56]. Antifungal activity of cadinene sesquiterpenes including cadinan-3-ene-2, 7-dione, 7-hydroxycadinan-3-ene-2-one, 5, 6- dihydroxycadinan-3-ene-2, 7-dione, cadinan-3, 6-diene-2, 7- dione and 2-acetyl-cadinan-3, 6-diene-7-one isolated from leaves of the plant were evaluated against four phytopathogenic fungi. These compounds were found to be selective against pathogenic fungi and the compound cadinan-3-ene-2, 7-dione showed the highest inhibitory action towards S. rolfsii (ED50 181.60 ± 0.58 μg/mL) and R. solani (ED50 189.74 ± 1.03 μg/mL) thus indicating significant antifungal activity of the plant [78]. Ahluwalia et al. analyzed the antifungal action of the essential oil of E. adenophorum against the pathogenic fungi, Sclerotium rolfsii, Macrophomina phaseolina, Rhizoctonia solani, Pythium debaryanum and Fusarium oxysporum. Among the five fungal strains tested, strongest inhibitory activity was found against S. rolfsii [35]. In another study, the essential oil from aerial parts exhibited moderate antifungal activity against Fusarium oxysporum by disk diffusion method [79].
Antitumour Activity
The cadenine sesquiterpene 9-oxo10, 11-dehydroageraphorone (euptox A) isolated from E. adenophorum was tested for cytotoxicity to human lung cancer A549 cells, Hela cells and Hep-2 cells in vitro. The results suggest that euptox A had significant antitumor activity against the three tumor cell lines in vitro in a dose-dependent manner. The percent inhibition of human lung cancer A549 cells, Hela cells and Hep-2 cells were 76.42, 68.30 and 79.05 %, respectively at concentration of 500 µg/mL treatment dose of Euptox A. The 50% inhibitory concentration (IC50) of euptox A for the three tumour cell lines were 369, 401 and 427 µg/mL (A549, Hela and Hep-2 cells, respectively. The results suggest better inhibitory action of euptox A than the control 5-FU thus proving it to be a low toxicity antitumor agent [69].
Cytotoxic Activity
In vitro trypanocidal activity of methanol extract of E. adenophorum leaves against Trypanosoma evansi on Vero cell line reported to display different cytotoxic effects such as distortion, swelling, sloughing and death of Vero cells compared to negative normal cells in the control ELISA plate wells [80]. The precise cytotoxicity of E. adenophorum in relation to the cell cycle and apoptosis of splenocytes in Saanen goats was studied which demonstrated that the plant significantly inhibits the growth of splenocytes through G0/ G1phase cell cycle arrest and the induction of apoptosis. E. adenophorum induced apoptosis and spleen impairment through the induction of mitochondrial dysfunction in splenocytes [81]. The cytotoxicity of ethanol extract of E. adenophorum leaf was tested against human breast adenocarcinoma cell line (MCF 7), human hepatocarcinoma cell line (HepG) and human cervix adenocarcinoma cell line (HeLa) by MTT assay, trypan blue exclusion assay. The leaf extract was found to inhibit the growth of HeLa cells by ~61%. An IC value of 32 μg/ml for HeLa cells and 50 μg/ml for HepG was obtained with the extract [70].
Pharmacology of Bioactive Compounds
E. adenophorum contains several chemical constituents with bioefficacy. Pharmacological studies on major sesquiterpenes including chlorogenic acid, neochlorogenic acid and cryptochlorogenic acid exhibited strong anti-inflammatory [82], anti-bacterial [83], and anti-obesity properties [84]. A large number of reports indicate that Euptox A, a cadenine sesquiterpene from E. adenophorum has wide range of biological activities such as antitumor [70], neurotrophic activity [85], larvicidal [86] and antiprotozoal [87] activities. The essential oil from aerial parts of the plant possesses insecticidal and antibacterial properties [35,39]. Flavonoids constituents of the plant have antioxidant and antibacterial bioactivities [88,89]. Polysaccharide constituents of leaves reported to have immunomodulatory properties and prophylactic effect against H5N1 influenza infection [43]. The main toxin of E. adenophorum , 9-oxo-10, 11- dehydroageraphorone can cause hepatotoxicity in mice [90] and rat [91]. Other cadinene sesquiterpenes such as 10Hα-9- oxo-ageraphorone and 10Hβ-9-oxo-ageraphorone also show toxicity to mice [92]. Essential oils from inflorescences, leaves, and roots of the plant have been investigated for their antimicrobial and antioxidant activities [30]. The oil showed strong inhibitory effect against bacteria, due to the presence of constituents such as camphene, p-cymene and α-terpinene, which has been proved to possess antimicrobial activity [93]. Pharmacological activities of the major bioactive chemical constituents of E. adenophorum are summarized in Table 1.
| Compounds | Plant part | Pharmacological activity | References Nos. |
|---|---|---|---|
| Chlorogenic acid | Leaves | Anti-inflammatory | [82] |
| Anti-bacterial | [83] | ||
| Anti-obesity | [84] | ||
| Neochlorogenic acid | Leaves | Anti-inflammatory | [82] |
| Anti-bacterial | [83] | ||
| Anti-obesity | [84] | ||
| Cryptochlorogenic acid | Leaves | Anti-inflammatory | [82] |
| Anti-bacterial | [83] | ||
| Anti-obesity | [84] | ||
| 5-O-trans-o-coumaroylquinic acid methylester | Aerial parts | Antibacterial,Antioxidant | [56] |
| Chlorogenic acid methyl ester | Aerial parts | Antibacterial, Antioxidant | [56] |
| Macranthoin F | Aerial parts | Antibacterial, Antioxidant | [56] |
| Macranthoin G | Aerial parts | Antibacterial, Antioxidant | [56] |
| Cadinan-3-ene-2,7-dione | Leaves | Antifungal | [78] |
| 7-hydroxycadinan-3-ene-2-one | Leaves | Antifungal | [78] |
| 5,6-dihydroxycadinan-3-ene-2,7-dione | Leaves | Antifungal | [78] |
| Cadinan-3,6-diene-2,7-dione | Leaves | Antifungal | [78] |
| 2-acetyl-cadinan-3,6-diene-7-one | Leaves | Antifungal | [78] |
| Euptox A (9-oxo10,11-dehydroageraphorone) | Leaves | Antitumor | [69] |
| Anticancer | [70] | ||
| Neurotrophic | [85] | ||
| Larvicidal | [86] | ||
| Antiprotozoal | [87] | ||
| Terpenoids | Leaves, Aerial parts | Antipyretic | [65] |
| Flavonoids | Leaves, Aerial parts | Antioxidant, Aantibacterial | [88,89] |
| Polysaccharide | Leaves | Immunomodulatory | [43] |
| Essential oil | Leaves | Insecticidal | [39] |
| Antibacterial | [35,39,56] | ||
| Antifungal | [35] | ||
| Antioxidant | [35,77] |
Table 1. Pharmacological activity of major bioactive compounds.
Toxicity
E. adenophorum has been reported to possess pneumotoxic and hepatotoxic effects in different species of animals. Consumption of E. adenophorum by horses results in chronic pulmonary disease in horses mainly in Australia, New Zealand, and the Himalayas [90,94]. Regular consumption of crofton weed by horses leads to chronic lung disease, known as Numinbah Horse Sickness or Tallebudgera Horse Disease in northern New South Wales and Queensland. However, no toxic effects were seen in goats with ingestion of plant from Nepal [95]. Experimental feeding of E. adenophorum plant growing in north-eastern India to cattle caused anorexia, suspension of rumination and photosensitization [96]. Methanolic extract of E. adenophorum leaf samples collected from Mizoram (India) has also been reported to induce hepatotoxicity in albino mice [97]. Pathological findings include pulmonary interstitial fibrosis and alveolar epithelisation. Exposure of mice to feed containing E. adenophorum freeze-dried leaf powder caused hepatotoxicity [98]. E. adenophorum leaf samples collected from Kangra Valley (India) and partially purified extracts from leaf samples mixed in the diet caused hepatotoxicity and cholestasis in rats [99,100].
Leaves and flowers of E. adenophorum contain substances such as butanedioic anhydride and 9-Oxo-10, 11- dehydroageraphorone (Euptox A) having strong local stimulation (especially at skin, mucosa and eyes) and even contact dermatitis [101]. It is proved that Euptox A is also a liver toxin [91,99], which may lead to icterus, bile duct hyperplasia and expansion, liver cell necrosis, an obvious increase of total bilirubin, alkaline phosphatase, aspartate aminotransferase and alanine aminotransferase. Seeds of the plant with huge amount of cilium may cause blindness especially in horses if floats into eyes [94]. The pollen and seed contain ageraphorone which cause allergic bronchial pneumonia in horses with the symptoms of acute pulmonary edema and subsequently cerebral hemorrhage resulting into the death [36]. The tannin, clavulanic alcohol and lactone can stimulate the mucosa of animal stomach, affecting the digestion system.
Conclusion
The interest in phytomedicine has been renewed over the few last decades and consequently, a number of plant species with traditional medicinal significance have been phytochemically and pharmacologically investigated in the quest of effective and safe herbal remedies. Most species of the genus Eupatorium are considered as weeds; as a result, very few plant species of the genus have been scientifically explored. Several plant species are yet phytochemically unstudied which might yield products of great therapeutic importance to humans. In recent years, Eupatorium adenophorum has received considerable attention and phytochemical and pharmacological studies of the plant have led to the isolation and characterization of a number of bioactive compounds as well as validation of its traditional medicinal uses. Phytochemical and pharmacological studies of extracts of different parts of E. adenophorum and compounds isolated from the plant have received much interest recently. A variety of bioactive chemical constituents and pharmacological activities, including anti-inflammatory, analgesic, antipyretic, antioxidant, antibacterial, antifungal, anticancer, and so on are presented in this review. Increasing amount of data supports application and exploitation of E. adenophorum for new drug development. However, due to certain known toxic effects of E. adenophorum , oral administration of the plant and its products without proper dosing should be avoided. Further, long-term toxicity and other toxicological aspects on E. adenophorum need to be investigated. The relationship of molecular structures of the bioactive compounds from E. adenophorum with its various pharmacological activities needs further study and confirmation.
Acknowledgement
The authors are grateful to the Director, Forest Research Institute, Dehradun for providing necessary facilities for carrying out this work. Authors are thankful to Prof. Lokesh Upadhyay, Department of Biotechnology, Sarmila Institute of Medicinal Products & Research Academy, Thanjavur, Tamil Nadu, India for his help in antifungal assay.
Conflict of Interest
Declared none.
References
- Gangwar KK, Deepali, Gangwar RS. Ethnomedicinal Plant Diversity in Kumaun Himalaya of Uttarakhand, India. Nature Sci. 2010; 8:66-78.
- Shrestha PM, Dhillion SS. Medicinal Plant Diversity and Use in The Highlands of Dolakha District, Nepal. J Ethnopharmacol. 2003; 86:81-96.
- Pushpangadan P. Biodiversity and Emerging Benefit Sharing Arrangements - Challenges and Opportunities for India. Proc Indian NatlAcad (PINSA). 2002; 68:297-314.
- Unial AK, Singh C, Singh B, Kumar M, Teixeira da Silva JA. Ethnomedicinal use of wild plants in Bundelkhand Region, Uttar Pradesh, India. J Med Aroma Plant SciBiotechnol. 2011;5: 81-86.
- Tripathi YC, Singh S. Prospecting Phytomedicinal Diversity: Threats and Challenges. In: Recent Progress in Medicinal Plants? Plant Bioactives in Traditional Medicine (Majumdar, D.K., Govil JN, Singh VK, Sharma RK Eds) USA: Studium Press LLC, Houston, Texas, 2005; 9:425-441.
- Katinas L, Gutierrez DG, Grossi MA, Crisci JV. PanÂorama de la FamiliaAsteraceae (= Compositae) en la República Argentina. BolSoc Argent Bot. 2007; 42:113-129.
- Zardini EM. Etnobotánica de CompuestasArgentinas Con Especial Referencia a suUsoFarmacológico (Primera parte). ActaFarmaBonaer. 1984;3:77-99.
- Rondina R, Bandoni A, Coussio J. Plantassilvestresargentinas con reconocidaspropiedadesmedicinales o tóxicas. OEA-CYTED:Buenos Aires (CD-ROM). 2003.
- Sasikumar JM, Doss PA, Doss A. Antibacterial Activity of Eupatorium glandulosum Leaves. Fitoterapia. 2005;70:240-243.
- Deciga Campos M, Rivero Cruz I, Arriaga Alba M, CastaÂñeda Corral G, Angeles Lopez GE, Navarrete A, Mata R. Acute Toxicity and Mutagenic Activity of Mexican Plants Used in Traditional Medicine. J Ethnopharmacol. 2007;110:334-342.
- Liu PY, Liu D, Li WH, Zhao T, Sauriol F, Gu YC, Shi QW, Zhang ML. Chemical Constituents of Plants from the Genus Eupatorium (1904-2014). ChemBiodivers. 2015; 12:1481-1515
- Zhang ML, Wu M, Zhang, JJ, Irwin D, Gu YC, Shi QW. Chemical Constituents of Plants from the Genus EupatoÂrium. ChemiBiodivers. 2008;5:40-55.
- Belter RK. Patent application title 20090100748: Petroleum substitutes from the essential oils of Eupatorium species, 2009.
- King RM, Robinson H. The Genera of the Eupatorieae (Asteraceae). Monographs in Systematic Botany. Missouri Bot. Gard. 22 St. Louis. Missouri Bot Gard. 1987; 22:581.
- Eupatorium adenophorum Spreng. Std Terms. in GBIF Secretariat. GBIF Backbone Taxonomy. Checklist dataset https://doi.org/10.15468/39omei 2017 accessed via GBIF.org on 2018-05-25.
- Zhou S, Tang C, Zhang X. The Damage Situation and Control Countermeasures for Eupatorium adenophorum in Sichuan Province. J Pratacult Sci. 2004;21:24?26.
- Duan H. Eupatorium adenophorumSpreng. Chin J Weed Sci. 2003;2:36-38.
- Sharma OP, Dawra RK, Kurade NP, Sharma PD. A Review of the Toxicosis and Biological Properties of the genus Eupatorium. Nature Toxins. 1998;6:1-14.
- Auld BA. The Distribution of Eupatorium adenophorumSpreng on the Far North Coast of New South Wales. J Proc R Soc New South Wales. 1969;102:159-161.
- Lu ZJ, Ma KP. Spread of the Exotic Croftonweed (Eupatorium adenophorum) across Southwest China along Roads and Streams. Weed Sci. 2006; 54:1068-1072.
- Sang WG, Zhu L, Axmacher JC. Invasion Pattern of Eupatorium adenophorumSpreng in Southern China. Biol Invasions. 2010;12:1721-1730.
- Baruah NC, Sarma JC, Sarma S, Sharma RP. Seed Germination and Growth Inhibitory Cadinenes from Eupatorium adenophorumSpreng. J Chem Ecol. 1994; 20:1885-1892.
- Wolff MA. Winning the war of Weeds: The Essential Gardener's Guide to Weed Identification and Control. Kenthurst, NSW: Kangaroo Press. 1999;17.
- Scher J. Federal Noxious Weed Disseminules of the U.S.:An Interactive Identification Tool for Seeds and Fruit of Plants on the United States Federal Noxious Weed List. CDROM. Animal and Plant Health Inspection Service (APHIS), United States Department of Agriculture (USDA). 2005.
- Cronk QCB, Fuller JL. Plant Invaders: The Threat to Natural Ecosystems. London: Chapman & Hall. 1995.
- Wang R, Wang YZ. Invasion Dynamics and Potential Spread of the Invasive Alien Plant Species Ageratinaadenophora (Asteraceae) in China. Divers Distrib. 2006;12:397-408.
- Bardoli MJ, Shukla VS, Sharma RP. Absolute Stereochemistry of The Insect AntifeedentCadinenes from Eupatorium adenophorum. Tetrahedron Lett. 1985; 26:509-510.
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. India: National Institute of Science Communication, New Delhi. 2006.
- Ansari S, Jain P, Tyagi RP, Joshi BC, Barar FSK. Phytochemical and Pharmacological Studies of the Aerial Parts of Eupatorium adenophorum L. HerbaPolonica. 1983;29: 93-96.
- Dahanukar SA, Kulkarni RA, Rege NN. Pharmacology of Medicinal Plants and Natural Products. Ind J Pharmacol. 2000;32:S81-S118.
- Kumar S. The Medicinal Plants of North-East India. India: Scientific Publishers. 2002; 88.
- Bantawa P, Rai R. Studies on Ethnomedicinal Plants Used by Traditional Practitioners, Jhankri; Bijuwa and Phedangma in Darjeeling Himalaya. Nat Prod Rad. 2009,8:537-541.
- Uprety Y, Poudel RC, Asselin H, Boon E. Plant biodiversity and Ethnobotany Inside the Projected Impact Area of the Upper Seti Hydropower Project, Western Nepal. Environ Dev Sustain. 2011;13:463-492.
- Awah FM, Uzoegwu PN, Ifeonu P, Oyugi JO, Rutherford J, Yao X, Fehrmann F, Fowke KR, Eze MO. Free Radical Scavenging Activity, Phenolic Contents and Cytotoxicity of Selected Nigerian Medicinal Plants. Food Chem. 2012;131:1279-1286.
- Ahluwalia V, Sisodia R, Walia S, Sati OP, Kular J, Kumdu A. Chemical Analysis of Essential Oils of Eupatorium adenophorum and Their Antimicrobial, Antioxidant and Phytotoxic Properties. J Pest Sci. 2014;87:341-349.
- Yan QS, Yang J, Li HM, Cao AC, Chen QH, Wen YQ, He L. Advances in the Studies on the Chemical Components and Bioactivity of Eupatorium adenophorumSpreng as a Intruding Species. J. Beijing Normal Univ. 2006;42:70-73.
- Li YM, Li ZY, Ye M. The Chemical Compositions and their Bioactivities in the Different Parts of Eupatorium adenophorumSpreng. J. Yunnan Agric Univ. 2008;23:42-46.
- He L, Hou J, Gan ML, Shi JG, Chantrapromma S, Fun HK, Williams ID, Sung HHY. Cadinanesesquiterpenes from the Leaves of Eupatorium adenophorum. J Nat Prod. 2008;71:1485-1488.
- Kurade NP, Jaitak V, Kaul VK, Sharma P. Chemical Composition and Antibacterial Activity of Essential Oils of Lantana camara, atumHoustonianum and Eupatorium adenophorum. Pharm Biol. 2010;48:539-544.
- Wei Y, Gao Y, Zhang K, Ito Y. Isolation of Caffeic Acid from Eupatorium adenophorumSpreng by High-Speed Countercurrent Chromatography and Synthesis of Caffeic Acid-Intercalated Layered Double Hydroxide. J LiqChromatogrRelat Technol. 2010;33:837-845.
- Wei Y, Zhang K, Zhang GL, Ito Y. Isolation of Five Bioactive Components from Eupatorium adenophorumSpreng Using Stepwise Elution by High-Speed Countercurrent Chromatography. J LiqChromatogrRelat Technol. 2011;34:2505?2515.
- Shi W, Luo SH, Li SH. Defensive Sesquiterpenoids from Leaves of Eupatorium adenophorum. Chin J Chem. 2012;30:1331-1334.
- Jin Y, Zhang YW, Wan CY, Wang HJ, Hou LY, Chang JY, Fan K, Xie XM. Immunomodulatory Activity and Protective Effects Of Polysaccharide from Eupatorium adenophorum Leaf Extract on Highly Pathogenic H5N1 Influenza Infection. Evid Based Complement. Altern. Med. 2013.
- Liu BY, Dong BT, Yuan XF, Kuang QR, Zhao QS, Yang M, Liu J, Zhao B. Enrichment and Separation of Chlorogenic Acid from the Extract of Eupatorium adenophorumSpreng by macroporous resin. J Chromatogr. 2016;1008:58?64.
- Yang GQ, Wan FH, Liu WX, Zhang XW. Physiological Effects of Allelochemicals from Leachates of AgeratinaadenophoraSpreng on Rice seedlSings. Allelopathy J. 2006;18:237-245.
- Zhao X, Zheng GW, Niu XM, Li WQ, Wang FS, Li SH. Terpenes from Eupatorium adenophorum and their Allelopathic Effects on Arabidopsis seeds germination. J Agric Food Chem. 2009;57:478?482.
- Zheng GW, Jia YX, Zhao X, Zhang FJ, Luo SH, Li H, Li WQ. o-Coumaric acid from Invasive Eupatorium adenophorum is a Potent Phytotoxin. Chemoecol. 2012;22:131-138.
- Bai J, Cao A, Guo M, Liu X, Liu X, Liang H, Zhou B. Identification of 9-oxo-10,11-Dehydroagerophorone in Eupatorium adenophorum by High Performance Liquid Chromatography. Chin Bull Bot. 2011;46:470?475.
- Xu YL, Shan XZ, Wang ZY. Chemical Constituents from Eupatorium adenophorum. Acta Bot Yunnan. 1998;10:238-240.
- Ding Z, Guo Y, Ding J. Chemical Constituents from the Flowers of Eupatorium adenophorum. ActaBotanicaYunnanica. 1999;21:505-511.
- Bohlmann F, Gupta RK. Six Cadinene Derivatives from Ageratinaadenophora. Phytochem. 1981;20:1432-1433.
- Lan H, Jie Y, Aocheng C, Yumei L, Yu A, Jiangong S. A New Sesquiterpenoid from Eupatorium adenophorumSpreng. Chin J Chem. 2006;24:1375-1377.
- Kundu A, Saha S, Ahluwalia V, Walia S. Plant Growth Inhibitory Terpenes from Eupatorium adenophorum Leaves. J Appl Bot Food Qual. 2013a;86:33-36.
- Zhang M, Liu WX, Zheng MF, Xu QL, Wan FH, Wang J, Lei T., Zhou ZY, Tan JW. Bioactive Quinic Acid Derivatives from Ageratinaadenophora. Molecules. 2013;18:14096-14104.
- Subba B, Kandel RC. Chemical Composition and Bioactivity of Essential Oil of Ageratinaadenophora from Bhaktapur District of Nepal. J Nepal Chem Soc. 2012;30:78-86.
- Pala-Paul J, Perez-Alonso MJ, Velasco-Negueruela A, Sanz J. Analysis by gas chromatography-mass spectrometry of the volatile components of AgeratinaadenophoraSpreng., growing in the Canary Islands. J Chromatography A. 2002;947:327-331.
- Padalia RC, Bisht DS, Joshi SC, Mathela CS. Chemical composition of the essential oil from Eupatorium adenophorumSpreng. J Essent Oil Res. 2009; 21:522-524.
- Padalia RC, Verma RS, Sundaresan V. Volatile constituents of three invasive weeds of Himalayan region. Rec Nat Prod. 2010;4:109-114.
- Weyerstahl P, Marschall H, Seelmann I, Kaul VK. Constituents of the flower essential oil of Ageratinaadenohpora (Spreng.) K.et R. from India. Flavour Frag J. 1997;12:387?396.
- Kritikar KR, Basu BD. Indian Medicinal Plants, Bishen Sing and Mahendra Pal Singh: Derhadun, India. 1987;3: 1331-1333, 1977-1978.
- Chakravarty AK, Mazumder T, Chatterjee SN. Antiinflammatory potential of ethanolic Leaf Extract of Eupatorium adenophorumSpreng through Alteration in Production of TNFa, ROS and Expression of Certain Genes. Evid Based Complement Alternat Med. 2011.
- Mandal SK, Boominathan R, Parimaladevi B. Dewanjee S, Mandal SC. Analgesic activity of methanol extract of Eupatorium adenophorumSpreng. leaves. Ind J ExptlBiol, 2005; 43: 662-663.
- Mandal SK, Mandal SC, Das AK, Tag H, Sur T. Antipyretic activity of Eupatorium adenophorum leaf extract. Ind J Nat. Prod 1981; 21: 6-9.
- Kundu A, Saha S, Walia S, Ahluwalia V, Kaur C. Antioxidant potential of essential oil and cadinenesesquiterpenes of Eupatorium adenophorum. Toxicol Environ Chem. 2013b; 95, 127-137.
- Pandey AK, Mohan M, Singh P, Palni UT, Tripathi NN. Chemical composition, antibacterial and antioxidant activity of essential oil of Eupatorium adenophorumSpreng. From Eastern UttarPradesh, India. Food Biosci. 2014; 7: 80-87/
- Singh YD, Mukhopadhayay SK, Shah MAA, Ali MA, Tolenkhomba TC. Effects of eupatorium adenophorum on antioxidant enzyme status in a mice model. Int J Pharm Pharm Sci. 2012; 4: 436-439.
- Liao F, Hu Y, Wu L, Tan H, Mo Q, Luo B, He Y, Deng J, Wei Y. Antitumor activity in vitro by 9-Oxo-10,11-dehydroageraphorone extracted from Eupatorium adenophorum. Asian J Chem. 2014;26:7321-7323.
- Tiwary BK, Bihani S, Kumar A, Chakraborty R, Ghosh R. The in vitro cytotoxic activity of ethnopharmacological important plants of Darjeeling district of West Bengal against different human cancer cell lines. BMC Complement Altern Med. 2015;15:22.
- Sasikumar JM, Doss PA, Doss A. Antibacterial activity of Eupatorium glandulosum leaves. Fitoterapia. 2005;70:240-243.
- Sobrinho ACN, de Morais SM, de Souza EB, and Fontenelle RODS. The genus Eupatorium L. (Asteraceae): A review of their antimicrobial activity. J Med Plants Res. 2017;11:43-57.
- Ringmichon CL, Gopalkrishnan B. Antipyretic activity of Eupatorium adenophorum leaves. Int J ApplBiolPharma Technol. 2017;8.
- Kumar N, Singh A, Sharma DK, Kishore K. Evaluation of Wound Healing Activity of Ageratinaadenophora (Spreng.) R.M.King&H.Rob. Int J Pharma Res Health Sci. 2017; 5: 1873-1876.
- Bhattarai N, Shrestha G. Antibacterial and Antifungal Effect of Eupatorium adenophorumSpreng against Bacterial and Fungal Isolates. Nepal J Sci Technol. 2009;10: 91-95
- Arvind N, Amit S. Antimicrobial Potential of Eupatorium adenophorumSpreng. Pharmacog J. 2011;2:61-64.
- Tian Yu, Hou Jing, Wu Jian-Ping, He Lan, Cao Ao-Cheng. Study on the volatile components from Eupatorium adenophorumSpreng and its antifungal activity. Chin J Pest Sci. 2007;2:49-52.
- Kundu A, Saha S, Walia S, Shakil NA, Kumar J, Annapurna K. Cadinenesesquiterpenes from Eupatorium adenophorum and their antifungal activity. J Environ Sci Health B. 2013c; 48:516-522.
- Chauhan N, Haider SZ, Lohani H, Godbole S, Gwari G, Bhandari U. Chemical Composition and Antifungal Activity of Essential Oil of Cymbopogondistans (Nees ex Steud.) W. Watson, Eupatorium adenophorumSpreng and Lantana camara L. grown in Uttarakhand (India). J Biol Active Prod Nat. 2015; 5:234-240.
- Shaba P, Pandey NN, Sharma OP, Rao JR, Mishra AK, Singh RK. Therapeutic activity of methanolic extract of Eupatorium adenophorum leaves against Trypanosomaevansi. Int J Basic Appl Med Sci. 2012; 2:77-82.
- He Y, Mo Q, Hu Y, Chen W, Luo B, Wu L, Qiao Y, Xu R, ZhouY, Zuo Z, Deng J, He W, Wei Y. E. adenophorum induces Cell Cycle Arrest and Apoptosis of Splenocytes through the Mitochondrial Pathway and Caspase Activation in Saanen Goats. Sci Rep. 2015;5:15967.
- Chagas-Paula DA, de Oliveira RB, da Silva VC, Gobbo-Neto L, Gasparoto TH, Campanelli AP, Faccioli LH, da Costa FB. Chlorogenic acids from tithoniadiversifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones. J Ethnopharmacol. 2011;136:355?362.
- Wang GF, Shi LP, Ren YD, Liu QF, Liu HF, Zhang RJ, Li Z, Zhu FH, He PL, Tang W. Anti-hepatitis b virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir Res. 2009;83:186-190.
- Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food ChemToxicol. 2010;48:937-943.
- Trzoss L, Xu J, Lacoske MH, Mobley WC, Theodorakis EA. Illiciumsesquiterpenes: divergent synthetic strat