Research Article - Biomedical Research (2017) Volume 28, Issue 18
A quick quantitative solution for the diagnosis of osteoporosis based on the conception of true bone density
Guodong Zhang1,2#, Xuanhuang Chen2#, Haibin Lin2, Zumei Xu2, Xu Chen2, Xiaohui Zheng3 and Wenhua Huang1*
1Department of Human Anatomy, School of Basic Medical Science, Southern Medical University, Guangzhou, Guangdong Province, PR China
2Department of Orthopedics, the Affiliated Putian Hospital of Southern Medical University, the Affiliated Hospital of Putian University, Putian, Fujian Province, PR China
3Department of Orthopedics, Hospital of Fujian Provincial Corps, Chinese People’s Armed Police Force, Fuzhou Fujian Province, PR China
#These authors contributed equally to this study
- *Corresponding Author:
- Wenhua Huang
Department of Human Anatomy
School of Basic Medical Science
Southern Medical University, PR China
Accepted on August 28, 2017
Abstract
Background: Accurate diagnosis of osteoporosis relies on precise measurement of bone mass and assessment of bone micro-architecture. However, the application of Bone Mineral Density (BMD) as the sole criterion of osteoporosis may not reflect all fractions of the bones. In this study, we retrospectively analysed the association between the mean CT value of different bone fractions and ages, and discussed the quantitative diagnosis of osteoporosis.
Methods: The thin-slice Computed Tomography (CT) scans of the lumbar vertebrae of 862 patients were retrospectively reviewed. The patients were divided into the Pathological Fracture (PF) group (n=107) and the Non-Pathological Fracture (NPF) group (n=755). The NPF group was further divided into 7 subgroups with a 10-year-increment. The vertebral bodies were divided into the overall vertebral bodies (Min-Max HU), the cancellous bone (Min-661HU) and the compact bone (662-Max HU) to investigate their mean CT values. The correlation between these parameters and age were analysed. The parameters of the PF group and the NPF subgroups were compared using the ANOVA analysis.
Results: The mean CT values of the overall vertebral bodies (R2, 0.76-0.79) and the cancellous bone (R2, 0.84-0.85) were negatively correlated with patient’s age, while that of the compact bone was only 0.25-0.36. There were significant differences in the mean CT values between PF group and the NPF subgroups (P<0.05).
Conclusion: The mean CT value of the cancellous bone, which reflect the true bone density, correlate well with the age, and might be promising candidates in the quantitative and differential diagnosis of osteoporosis and the bone fracture risk assessment.
Keywords
Thin slice CT scan, Bone density, Bone mineral density, Osteoporosis, CT value.
Introduction
Osteoporosis is characterized by loss of bone mass, deterioration of bone micro architecture, decreased bone strength and the consequent increased risk of pathological fractures [1]. Globally, the increased incidence of osteoporosis is largely attributes to the increased proportion of aging population [2-4]. The treatment of osteoporosis and the associated pathological fractures impose a significant cost. Early diagnosis and timely treatment of osteoporosis can help improve the prognosis. The Bone Mineral Density (BMD) measured with a dual energy X-ray absorptiometry (DXA) is the gold standard for diagnosing and evaluating the efficacy of osteoporosis treatment [5-11]. However, the application of BMD as the sole criterion for diagnosis of osteoporosis has often been questioned [12-14]. Various factors such as hyperosteogeny, body shape, and the position of the patient during detection may affect the BMD evaluation. Moreover, its interpretation is also subject to considerable inter-observer variability depending on the experience of the evaluator.
QCT can quantitatively examine the BMD by using the phantom. However, the selection of the highly homogeneous areas of interest is a challenging task [15] that requires considerable time and effort in partitioning the images [16]. The final evaluation of QCT remains centered solely on the BMD.
The mean CT values obtained by common CT scanners have been used to quantify the BMD of any selected area of interest [17]. The CT value was determined by the true bone density but not the BMD. In terms of the diagnosis of osteoporosis, is it necessary to use the switched BMD parameter? In the present study, we aimed to evaluate the mean CT value of the lumbar vertebrae using CT images and to establish a convenient method for the diagnosis of osteoporosis.
Materials and Methods
Patients
Thin slice CT radiographs of the lumbar vertebrae of 862 patients treated from April 2012 to August 2015 at our hospital were retrospectively reviewed. The scanning parameters were 120 kv, 264 mA, and pitch 0.625 mm (Light Speed, GE Medical Systems, USA).
The patients were divided into two group: the Pathological Fracture (PF) group (n=107) and the Non-Pathological Fracture (NPF) group (n=755). The NPF group included 300 males and 455 females with a mean age of 46.9 y (range, 14-92 y). The NPF group was categorized into subgroups according to their age, i.e., <21 y, 21-30 y, 31-40 y, 41-50 y, 51-60 y, 61-70 y, and >70 y. The PF group included 35 males and 72 females with a mean age of 62 y (range 42-88 y). Patients with traumatic fractures were excluded from this study. A total of 2435 vertebrae (L3-L5) in patients without pathological fractures and 342 vertebrae in those with pathological fractures came within the purview of our analysis.
The edition of CT images
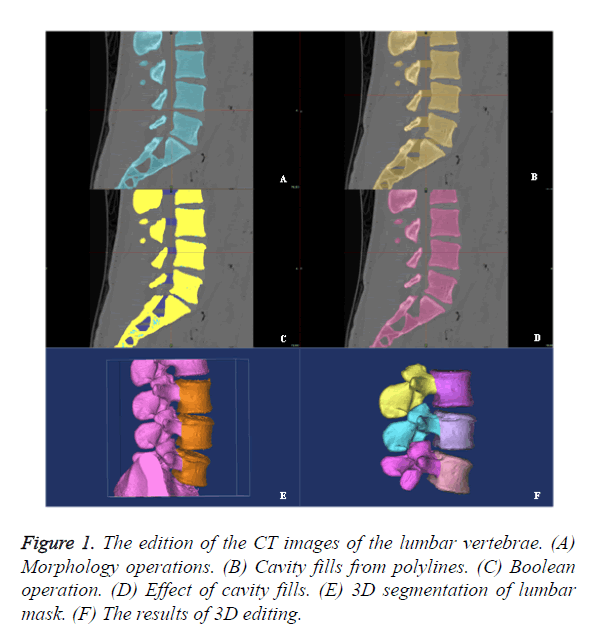
The CT images of the lumbar vertebrae were transferred into the workstation (Dell T7500) for three-dimensional reconstruction using the Mimics 14.0 software (Materialise, Belgium). Mask A was obtained after setting mimics with threshold value of 148-maximum HU with region growing, morphology operation, number of pixels of 3, operation at close and 8-connectivity. Mask B was then obtained by applying calculate polylines and cavity fill from polylines. Mask C was obtained through boolean operation (Mask C=Mask B-Mask A). Mask D was the ideal mask with perfect cavity filling without wrong selection. Mask E was the 3D image segmentation in 3D edit windows by the method of edit mask in 3D. Edit mask in 3D could be used as a powerful tool for the quick segmentation of lumbar vertebra. The final result of the 3D edition was obtains anterior vertebral body and posterior structure, respectively (Figure 1). All CT values in the vertebral body were completely selected by the above operations.
Diagnosis criteria of osteoporosis
The diagnosis criteria of osteoporosis were according to the International Society for Clinical Densitometry (ISCD) in 2007 and American College of Radiology (ACR) in 2013.
Normal: Absolute value of bone mineral density ≥ 120 mg/ cm3;
Bone loss: 80 ≤ Absolute value of bone mineral density<120 mg/cm3;
Osteoporosis: Absolute value of bone mineral density<80 mg/cm3.
Data analysis
The CT data was exported from the 3D images as text files. The CT values were automatically divided into 3 different components using macro command of Excel 2010: (1) overall vertebral body including values of all pixels of overall vertebral body (Min-Max HU); (2) cancellous bone (Min-661HU); (2) compact bone (662-Max HU). The macro command was also able to automatically calculate the average value of these 3 components, that is, the mean CT value.
Statistical analysis
SPSS 19.0 software was used for all analyses. Statistical significance was defined as p<0.05. (1) Correlation and regression analysis: the relationship between the mean CT value (overall vertebral body, cancellous bone and compact bone) and age; (2) ANOVA test: the CT parameters of the PF group and the NPF subgroups were compared using the ANOVA test.
Results
Correlation and regression analyses
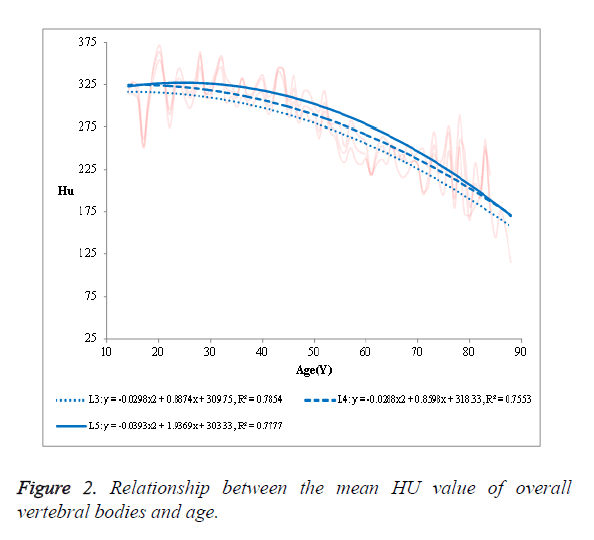
(1) The mean HU value of the overall vertebral bodies in the NPF group showed a negative correlation with age (Figure 2).
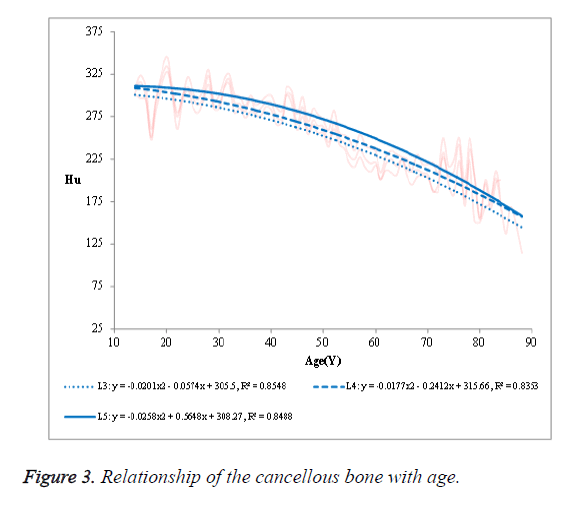
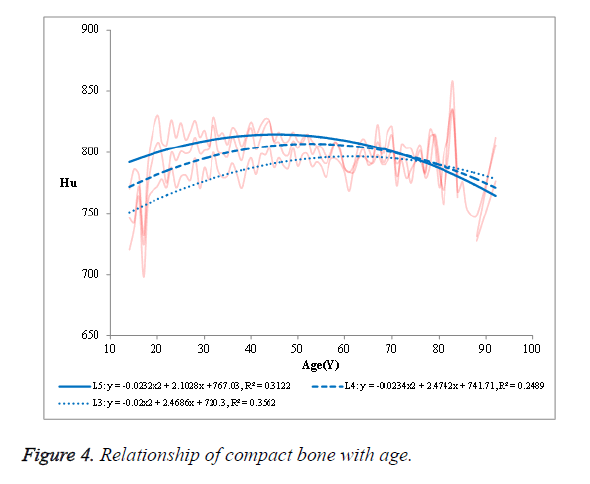
The R2 was 0.7854 in L3, 0.7553 in L4 and 0.7777 in L5, respectively. (2) The mean HU value of the cancellous bone in the NPF group showed a negative correlation with age (Figure 3). The R2 was 0.8548 in L3, 0.8353 in L4 and 0.8458 in L5, respectively. (3) No significant correlation was observed in case of compact bone (Figure 4).
One-way ANOVA
The mean and standard deviation of the PF group and that of the age-subgroups of the NPF group are listed in Table 1. Significant differences were found in the mean CT values of the overall vertebral body and cancellous bone between the PF group and the age-subgroups of the NPF group (all P=0).
| PF group | NPF group | |||||||
|---|---|---|---|---|---|---|---|---|
| <21 y | 21-30 y | 31-40 y | 41-50 y | 51-60 y | 61-70 y | >70 y | ||
| Cancellous bone | 132.16 ± 22.41 | 302.04 ± 31.61 | 297.44 ± 41.3 | 291.5 ± 41.48 | 275.55 ± 41.14 | 239.36 ± 38.46 | 217.25 ± 44.41 | 205.82 ± 43.66 |
| Overall vertebral body | 168.22 ± 37.05 | 316.23 ± 38.42 | 320.77 ± 47.95 | 320.15 ± 51.37 | 305.78 ± 53.96 | 264.5 ± 50.55 | 241.6 ± 60.02 | 227.65 ± 55.15 |
| ANOVA: Analysis of variance; HU: Hounsfield Unit. | ||||||||
Table 1. One-way ANOVA of HU values in the PF group and the age-subgroups of the NPF group (mean ± standard deviation).
Discussion
In this paper, we discussed the feasibility for the diagnosis of osteoporosis by using the parameter of mean value of CT obtained from the thin-slice CT scan. This study focused on 3 key issues: (1) whether the CT value of bone had a good correlation with age; (2) whether the CT value of the definite osteoporosis patients who suffered from the pathological fracture had a significant difference while comparing with the other cases; (3) by what method a diagnostic index with a good quality control could be harvested. The CT value of bone is determined by its true bone density basically, therefore, in this sense this paper aims to investigate whether the true bone density value can be used for the quantitative diagnosis of osteoporosis.
Our study showed that the mean HU value of the overall vertebral bodies and that of the cancellous bone were negatively correlated with age. However, the R2 of the cancellous bone with age (0.8353, 0.8458 and 0.8548) was better than that of the overall vertebral bodies (0.7553, 0.7777 and 0.7854). Obviously, the mean HU value of the cancellous bone was more easily used to the relevance with age than that of the overall vertebral bodies, in other words, the former had a better diagnostic value than the latter. No significant correlation was observed in case of compact bone with age.
Our study also showed that there were significant differences in the mean CT values between PF group and the NPF subgroups whether in the overall vertebral bodies group or the cancellous bone (all p<0.05). These results revealed that the parameter of the mean CT value might be promising candidates for the quantitative and differential diagnosis of osteoporosis. Table 1 showed that the PF group was significant lower than the NPF subgroups. This revealed that the parameter of the mean CT value could be used as an effective tool for the bone fracture risk assessment.
The mean HU value of the vertebral body appeared to decrease with an increase in age. This may be due to the loss of bone mass associated with ageing. Compared with the correlation between the cancellous bone and age, the correlation was less significant between the mean HU value of the overall vertebral bodies and age, this discrepancy could be caused by hyperosteogeny. In addition, with age increasing, there are two accompanying processes including bone hyperplasia and bone mass loss. For the reason that the bone hyperplasia does not affect the loss of cancellous bone, the CT value of cancellous bone has a better correlation with age. The cancellous bone is the predominant component of the vertebral body volume, and is also the primary site of bone mass loss from the vertebrae. This explains the significant correlation of the mean CT value of the cancellous bone with age (R2, 0.8048-0.8822).
No significant correlation was found between the mean CT value of the compact bone and age (R2, 0.2489, 0.3122 and 0.3562). Furthermore, the mean CT value of the compact bone were significantly higher in patients approaching 50 y of age, followed by a decrease with a further increase in age (Figure 4); presumably because that the compact bone mass loss was higher than the amount of hyperosteogeny in patients over 50 y old.
In the current study, we used methods like DXA and QCT for measuring BMD. However, these two methods have their own inherent limitations which may lead to a false diagnosis.
The issues include: (1) Degenerative hyperosteogeny may increase BMD rather than decreasing it; (2) The bone micro architecture deterioration was very severe [18-20]; (3) The dual energy X-ray absorptiometry measures the BMD in a two-dimensional manner; (4) The accuracy of the DXA is often compromised when the patient fails to meet the standard position due to any morbid condition. Moreover, patient’s body shape may also affect the DXA results [21]. Moreover, it is difficult to ensure the high homogeneity of the area of interest for DXA examination [22]. Some studies have demonstrated an association between osteoporosis and obesity. Therefore, the effect of obesity on the DXA results is a potential confounding influence [23]. In QCT examination, the area of interest is arbitrarily selected rather than the entire vertebral body, which may introduce selection bias and affect the results of BMD. Besides, the change in patient’s position during the QCT examination is usually a hurdle for maintaining a highly homogenous area of interest [15]. The areas of interest for the cancellous bone and the compact bone are also arbitrarily partitioned for QCT examination. This partitioning procedure can be affected by human eye resolution and, therefore, has a low precision [16].
We analysed the thin-slice CT data to evaluate the mean CT value of difference fractions, including the overall vertebral bodies, the cancellous and the compact bone. All these parameters are decided by the true bone density of the different fractions. In this study, we proposed that the conception of the osteoporosis can also achieve rapid quantitative diagnosis by real bone mineral density, in addition to BMD.
The mean HU value of the bone reflects all the bone contents and the real bone density, which is representative of the bone strength [24]. The analysis of changes of different bone fractions is more advantageous for the evaluation of bone structures.
The dual energy X-ray absorptiometry lacks the ability to assess bone micro-architecture due to its two-dimensional property. Trabecular Bone Score (TBS) is usually used to assess the fracture risks in a qualitative rather than quantitative perspective [25,26]. Other factors, such as operative errors, patient positioning and the hyperosteogeny also affect the ability of two-dimensional DXA in assessing three-dimensional bone micro architecture. Even the QCT examination cannot fully evaluate the bone mass contents and the bone micro architecture. The three-dimensional QCT overcomes the errors associated with the two-dimensional DXA examination, thus making it superior to DXA, especially for evaluating degenerative changes in the bone [27]. Additionally, the QCT can also help assess the optimization and expansion of the trabecular bone after treatment [28]. However, QCT is limited by its arbitrary selection of the area of interest, and thereby does not reflect the entire bone content and its micro architecture. Therefore, the common CT can be used to establish a diagnosis of osteoporosis and appears to be superior to DXA in this respect [29]. The images obtained from the common CT and the area BMD are used to screen the osteoporosis with a high level of accuracy [30]. Sometimes, common CT without phantom is also used to evaluate BMD [31,32] for the reason that it can intuitively demonstrate the BMD values using different colors [33]. In addition, the images obtained from common CT can be converted into BMD and further used to predict the risk of fracture of the vertebral body [34].
The mean CT values obtained from the thin slice scanning can better reflect the absolute true bone density when compared to a single BMD. According to available evidence, the mean CT values can be used for evaluating BMD and bone strength [35], and also for diagnosing of osteoporosis [36]. However, the mean CT values may significantly vary between the elderly and younger patients [37,38] and also differ with sex [17]. The earlier studies have noticed the association between the age and mean CT values, and thus have tried to evaluate the true bone density using mean CT values. But a single mean CT value of the overall vertebral bodies does not suffice for the evaluation of bone micro architecture. In our present study, the mean CT value of cancellous bone might be a better parameter for quantitative judgment of bone structure.
Although the previous studies failed to address the quality control aspect of the protocol, our method uses 3D editing and morphology manipulation to select an area of interest with a high homogeneity. This is an effective method with high quality control for the rapid diagnosis of osteoporosis.
Conclusion
The mean CT value of the cancellous bone, which reflects the true bone density, correlate well with the age, furthermore, might be promising candidates for the quantitative and differential diagnosis of osteoporosis and the bone fracture risk assessment.
Acknowledgements
This study was supported by the following grants: Natural Science Foundation of Fujian Province (Grant number: 2014J01377), Natural Science Foundation of Fujian Province (Grant number: 2013J01391), the Project of Science and Technology of Putian City of Fujian Province (Grant number: 2012S03 (4)).
References
- Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 2008; 371: 1505-1512.
- Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 2014; 29: 2520-2526.
- Hou K, Lin C, Chen C, Wu B, Zhu D, Zhong W, Wang X, Xie X, Chen Q. Association of probiotics and bone mineral density in Chinese patients with type 2 diabetes. Biomed Res 2017; 28: 129-133.
- Shiraki M, Kuroda T, Shiraki Y, Aoki C, Sasaki K. Effects of bone mineral density of the lumbar spine and prevalent vertebral fractures on the risk of immobility. Osteoporos Int 2010; 21: 1545-1551.
- World health organization assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series 843. WHO Geneva 1994.
- Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Intl 1994; 4: 368-381.
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7-29, 2000: highlights of the conference. South Med J 2001; 94: 569-573.
- Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P. Predictive value of BMD for hip and other fractures. J Bone Miner Res 2005; 20: 1185-1194.
- Sestak I, Singh S, Cuzick J, Blake GM, Patel R. Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: an international, double-blind, randomised, placebo-controlled trial. Lancet Oncol 2014; 15: 1460-1468.
- Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2009; 373: 1253-1263.
- Weycker D, Lamerato L, Schooley S, Macarios D, Siu Woodworth T. Adherence with bisphosphonate therapy and change in bone mineral density among women with osteoporosis or osteopenia in clinical practice. Osteoporos Int 2013; 24: 1483-1489.
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd ed.). Autophagy 2016; 12: 1-222.
- Sun X, Yuan X, Chen L, Wang T, Wang Z, Sun G, Li X, Li X, Liu G. Histamine induces bovine rumen epithelial cell inflammatory response via NF-κB pathway. Cell Physiol Biochem 2017; 42: 1109-1119.
- Kanis JA, Black D, Cooper C, Dargent P, Dawson-Hughes B. A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int 2002; 13: 527-536.
- Karlsson MK, Linden C, Karlsson C, Johnell O, Obrant K, Seeman E. Exercise during growth and bone mineral density and fractures in old age. Lancet 2000; 355: 469-470.
- Marjanovic EJ, Ward KA, Adams JE. The impact of accurate positioning on measurements made by peripheral QCT in the distal radius. Osteoporos Int 2009; 20: 1207-1214.
- Mastmeyer A, Engelke K, Fuchs C, Kalender WA. A hierarchical 3D segmentation method and the definition of vertebral body coordinate systems for QCT of the lumbar spine. Med Image Anal 2006; 10: 560-577.
- Xuan PF, Zhang G, Huang W, Ke L, Huang H, Li S. A quick solution for lumbar vertebra CT values in different ages and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao 2014; 34: 1799-1803.
- Atalay A, Kozakcioglu M, Cubuk R, Tasali N, Guney S. Degeneration of the lumbar spine and dual-energy X-ray absorptiometry measurements in patients without osteoporosis. Clin Imaging 2009; 33: 374-378.
- Bauman WA, Schwartz E, Song IS, Kirshblum S, Cirnigliaro C, Morrison N. Dual-energy X-ray absorptiometry overestimates bone mineral density of the lumbar spine in persons with spinal cord injury. Spinal Cord 2009; 47: 628-633.
- Tenne M, McGuigan F, Besjakov J, Gerdhem P, Akesson K. Degenerative changes at the lumbar spine-implications for bone mineral density measurement in elderly women. Osteoporos Int 2013; 24: 1419-1428.
- Boyanov M. Estimation of lumbar spine bone mineral density by dual-energy X-ray absorptiometry: standard anteroposterior scans vs. sub-regional analyses of whole-body scans. Br J Radiol 2008; 81: 637-642.
- Greco EA, Fornari R, Rossi F, Santiemma V, Prossomariti G, Annoscia C. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int J Clin Pract 2010; 64: 817-820.
- Lee HR, Hong SS, Lee SY, Cho YH, Park HJ, Jung DW. The impact of body weight change on bone mineral density of the lumbar spine in perimenopausal women: A retrospective, one-year follow-up study. Korean J Fam Med 2011; 32: 219-225.
- Schileo E. An accurate estimation of bone density improves the accuracy of subject-specific finite element models. 2008; 41: 2483-2491.
- Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N. Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese Population-Based Osteoporosis (JPOS) cohort study. J Bone Miner Res 2014; 29: 399-407.
- Pothuaud L, Barthe N, Krieg MA, Mehsen N, Carceller P, Hans D. Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study. J Clin Densitom 2009; 12: 170-176.
- Li N, Li XM, Xu L, Sun WJ, Cheng XG, Tian W. Comparison of QCT and DXA: Osteoporosis Detection Rates in Postmenopausal Women. Int J Endocrinol 2013; 2013: 895474.
- Engelke K, Fuerst T, Dasic G, Davies RY, Genant HK. Regional distribution of spine and hip QCT BMD responses after one year of once-monthly ibandronate in postmenopausal osteoporosis. Bone 2010; 46: 1626-1632.
- Pickhardt PJ, Lee LJ, Del RA, Lauder T, Bruce RJ, Summers RM. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res 2011; 26: 2194-2203.
- Tay WL, Chui CK, Ong SH, Ng AC. Osteoporosis screening using areal bone mineral density estimation from diagnostic CT images. Acad Radiol 2012; 19: 1273-1282.
- Mueller DK, Kutscherenko A, Bartel H, Vlassenbroek A, Ourednicek P, Erckenbrecht J. Phantom-less QCT BMD system as screening tool for osteoporosis without additional radiation. Eur J Radiol 2011; 79: 375-381.
- Du XL, Shi Z, Peng ZC, Zhao CX, Zhang YM, Wang Z, Li XB, Liu GW, Li XW. Acetoacetate induces hepatocytes apoptosis by the ROS-mediated MAPKs pathway in ketotic cows. J Cell Physiol 2017; 9999: 1-13.
- Wichmann JL, Booz C, Wesarg S, Kafchitsas K, Bauer RW, Kerl JM. Dual-energy CT-based phantomless in vivo three-dimensional bone mineral density assessment of the lumbar spine. Radiology 2014; 271: 778-784.
- Schwaiger BJ, Gersing AS, Baum T, Noel PB, Zimmer C, Bauer JS. Bone mineral density values derived from routine lumbar spine multidetector row CT predict osteoporotic vertebral fractures and screw loosening. AJNR Am J Neuroradiol 2014; 35: 1628-1633.
- Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am 2011; 93: 1057-1063.
- Lee S, Chung CK, Oh SH, Park SB. Correlation between bone mineral density measured by dual-energy x-ray absorptiometry and Hounsfield units measured by diagnostic CT of lumbar spine. J Korean Neurosurg Soc 2013; 54: 384-389.
- Emohare O, Cagan A, Morgan R, Davis R, Asis M, Switzer J. The use of computed tomography attenuation to evaluate osteoporosis following acute fractures of the thoracic and lumbar vertebra. Geriatr Orthop Surg Rehabil 2014; 5: 50-55.