Research Article - The International Tinnitus Journal (2025) Volume 29, Issue 1
A Preliminary study to explore the air Caloric test using Videonystagmography Among Neurotypical Adults And Individuals With Congenital Blindness
Assistant Professor, Bangalore Speech and Hearing Research Foundation, Chandrasekhar institute of Speech and Hearing, Bangalore, India
Send correspondence to:
Srividya A
Assistant Professor, Bangalore Speech and Hearing Research Foundation, Chandrasekhar institute of Speech and Hearing, Bangalore, India, Email:
the.srividya@gmail.com
Tel: 9880938138
Paper submitted on January 29, 2024; and Accepted on Feb 25, 2024
Citation: Srividya A. A Preliminary study to explore the air Caloric test using Videonystagmography among Neurotypical Adults and Individuals with Congenital Blindness. Int Tinnitus J. 2024;29(1): 22-32.
Abstract
Background and Justification: These are limited studies that have documented normative data in the Indian population. As the values can vary depending on the instrumentation used and other variables, each laboratory is recommended to have its own norms (BSA, 2006). Therefore, the present study was undertaken. Aim: Air caloric using videonystagmography among neurotypical adults and individuals with congenital blindness (preliminary study). Method: To obtain representative SPV values for cold and warm irrigation (air) among neurotypical young adults for two different duration of caloric stimulation (75sec and 60 sec). To obtain representative SPV values for cold and warm irrigation (air) among congenitally blind individuals (adults) for two different durations of caloric stimulation (75sec and 60 sec). To compare SPV values for warm irrigation of 75 sec duration and 60sec duration among neurotypical young adults. To compare SPV values for Cold irrigation of 75 sec duration and 60sec duration among congenitally blind individuals Results: The outcome of the study aimed to compare SPV values for Cold irrigation of 75s duration and 60s duration among neurotypical young adults. The result revealed that there was a significant difference for the measured SPV ( table 2) between the 75s and 60s stimulation of air caloric. This significant difference was observed in RW, RIGHT BEATING, and LEFT BEATING for stimulation of air caloric among the participants of neurotypical young adults. The values were higher for 75s, and the duration was well tolerated by all participants. Therefore, it is recommended for routine clinical use. And for next objective there is no significant difference on RW, LW, RC & LC between the neurotypical group and individuals with congenital blindness (p > 0.05).
Introduction
The vestibular system differs from other sensory systems in that it operates largely in the service of motor reflexes outside the field of conscious perception. Vestibulo-Ocular Reflex (VOR) helps keep the gaze steady during head and body movement. It is accurate enough to maintain the velocity of the eye in tandem with the velocity of the head during any movement over a long range of velocities of movement [1].
The vestibulo-ocular reflex is examined with the caloric reflex test (vestibular caloric stimulation), which involves irrigating the external auditory canal with cold or warm water or air and recording eye movements for nystagmus. The test was developed the to evaluate the performance of a single vestibular end organ at a time and offered a physiologic theory for the response brought on by thermal irrigation [2]. Any angular head movement in any direction will naturally excite the Vestibulo-Ocular Reflex (VOR). The VOR is naturally activated by rotating stimuli; however, this activation always excites one side while inhibiting the other member of the functional pair on the opposite side, so it was necessary to find a way of isolating one peripheral at a time.
The thermal caloric test is efficient to isolate and evaluate one peripheral vestibular apparatus at a time. Though other tests have evolved over time, that isolate the peripheral vestibular system components for evaluation, such as the Video Head Impulse Test (vHIT) for the semi-circular canals [3-6] and the ocular and cervical Vestibular-Evoked Myogenic Potential (VEMP) for the otolith organs [7], the caloric test allows for an estimation of the strength of the response that can be used without moving the head [8,9]. It enables examinations of patients with limited cervical range of motion while they are still in bed. In comatose patients, the thermal caloric test is also performed to get a general idea of how well the brainstem is working. Since the test's inception, there have been several limitations to its performance [10,11]conducted a study on the sensitivity of caloric tests and video head impulses as screening tests for chronic vestibular complaints among 157 subjects with complaint of dizziness with vestibular characteristics of varied durations and clinical courses. They expressed that the caloric test and video head impulse test results are distinct yet complementary and the caloric test was more sensitive for vestibular impairment unless chronic conditions.
Caloric evaluation mostly assesses the horizontal semi-circular canal. A heat stimulus in the external auditory canal has very little impact on the vertical canals because of the anatomical layout of the vertical canals and their different distances from the external auditory canal. As a result, the horizontal canal is primarily responsible for the reaction to the heat gradient across the middle ear cleft into the vestibular labyrinth [12].
The frequency of the rotational stimulus required to generate the Slow Phase Velocity (SPV) can be determined as it is the half-cycle of the rotational stimulus, which is less than 0.003 Hz, ranging from 0.003 to 0.008 Hz, secondary to the individual heterogeneity in the capacity of the temperature gradient to drive the horizontal canal [13]. The significance of this restriction on the caloric test is that it does not necessarily follow that the peripheral is completely devoid of function if the caloric test produces no reaction for warm, cool, or icy water on a specific side. Only the horizontal canal's very-low-frequency portion was assessed during the test.
By generating a temperature differential from one side of the horizontal semi-circular canal to the other, the caloric test causes endolymphatic flow and horizontal nystagmus [14]. The temperature of the endolymph column closest to the middle ear lowers in response to a cold caloric stimulation, and it moves inside the canal because of its increased density. This causes the cupula to diverge from the utricle (ampullofugal flow) and results in horizontal nystagmus with the fast phase directed away from the stimulated ear. Warm stimulation has the opposite effect, generating ampullopetal endolymph flow towards the stimulated ear and nystagmus with its fast phase towards the stimulated ear. A mnemonic used for this is COWS, which stands for "Cold Opposite, Warm Same".
The inner ear is a fluid-filled cavity within the temporal bone of the skull and is comprised of the semi-circular canals (h = horizontal; p = posterior; a = anterior) and the otolithic organs (s = saccule; u = utricle), as well as the cochlea. (B) The caloric test irrigates the ear canal with warm (or cold) water while the head is positioned so that the horizontal canal is roughly in a vertical plane. (C) Thermal stimulation is thought to create a temperature gradient across the horizontal canal, creating an upward convection force against gravity if warm (left) or downward if cool (right), stimulating the cupula in the canal (Figure1).
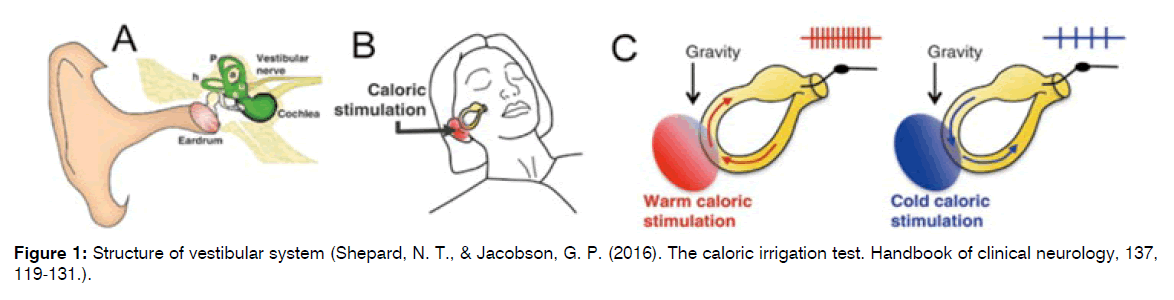
Figure 1: Structure of vestibular system (Shepard, N. T., & Jacobson, G. P. (2016). The caloric irrigation test. Handbook of clinical neurology, 137, 119-131.).
The horizontal canal practically extends into the middle ear space, as shown in Figure 1, and is quite close to the ear canal. This explains why using cold water instead of warm or positioning the patient upside-down causes the nystagmus to reverse, beating in the opposite direction. Since the horizontal canal responds to rotations in this plane, it also explains why the eyeballs beat horizontally, from left to right.
Knowing that the semi-circular canals respond more efficiently to angular movements at 1 to 6Hz, it may be concluded that caloric testing assesses the labyrinth at a non-physiological frequency. Head velocity during normal daily head and body movements is higher, in the range of 3Hz to 30 Hz. The order in which the four caloric irrigations are performed is not yet uniformly followed across all clinical and lab facilities, but generally it is done by carrying out warm and cold irrigation in one ear and moving to warm and cold irrigation in another ear.
Caloric stimulation may be done with water or air as they generate nystagmus in the intact vestibular system hence appropriate for clinical testing. Caloric generates an endolymphatic current within the stimulated lateral canal, analogous to a 0.003 Hz angular movement [15]. Many studies [16,17] later confirmed that water stimulation induces more robust caloric responses and causes less variability among individuals compared to air stimulation. On the other hand, water stimulation produces more frequent neuro-vegetative reactions compared to air stimulation16.
In classical studies of Barany water caloric stimulation was used. The temperature settings and duration of stimulation were standardized for water irrigation by Fitzgerald and Hallpike. They recommended irrigating the ear with water for 40 seconds at temperatures of 44°C and 30°C, 7°C over and 7°C below the human body temperature, which generates the endolymphatic current. The Barany Society gave recommendations for air and water caloric testing (2008), which may help in standardizing the test protocol across clinics. In air stimulation, 8 l/min air current is administered for 60 seconds at 50°C and 24°C (13°C above and 13°C below body temperature), creating an endolymphatic current that is comparable to that produced by water at 44°C and 30°C. Changes in the stimulation depth within the external auditory canal may lessen the slow phase of the nystagmus by 20% to 40% since air does not transfer heat well [18]. As a result, this method requires more technical know-how than caloric stimulation. Other stimulation standards have also been employed, including 45.5°C and 27.5°C for 100 seconds at a flow rate of 13 l/min or 18°C and 42°C for 80 seconds at a rate of 7–8 l/min [19].
Caloric testing evaluates the functioning of semi-circular canals with the help of measurable parameters such as SPV, duration of nystagmus, latency of nystagmus appearance, and fixation suppression of nystagmus. With the help of the normative values of the parameters, we can identify unilateral vestibular deficits, bilateral vestibular deficits, and central causes of vestibular symptoms. Responses are compared to normative data in order to classify them as normal or abnormal. In absolute values of SPV, nystagmography responses can be hypereflexia, i.e higher than expected, hyporeflexia, i.e., responses lower than expected, and arreflexia, i.e. absence of a caloric response.
Unilateral weakness (%) is calculated by applying Jonkeeys formula.
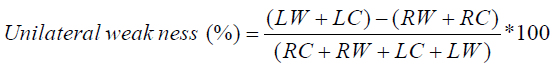
Fixation suppression, i.e., the change in the nystagmus parameter when eyes are fixed in gaze during the caloric testing, is measured for each of the four caloric responses. (e.g., right warm, left warm) with a time duration of 10-15 seconds. This measure helps in differentiating between peripheral and central causes of vestibular disorders. Abnormal results on caloric testing are seen in brain injury, acoustic nerve damage, harm to the balancing sensors in your ears, and aberrant eye movements, in the ear's with blood flow weakened by atherosclerosis and various pollutants, which causes trauma bleeding to clot, problematic blood vessels and issues with bleeding tumours in the ears are brought on by rubella. Medication like salicylates, antibiotics, and medications to prevent malaria, can affect the ear nerves The outcomes of this test may also be used to rule out or support particular diagnoses, including, Meniere's disease and benign positional vertigo caused by labyrinthitis or an acoustic neuroma.
The most common factors that influence the results of caloric are temperature and humidity [20], method of irrigation (water vs. air), duration of stimulation, and position of head (supine vs. prone) [21]. Water stimulation induces more robust caloric responses and causes less variability among individuals compared to air stimulation [22]. But water stimulation also produces more frequent neuro-vegetative reactions compared to air stimulation [23].
In cases of congenital blindness, individuals may be dependent on other intact sensory systems. They usually depend on the inner ear for better auditory processing and vestibular processing in order to move around and maintain balance. Of the three sensory inputs that regulate balance in neurotypical individuals, the visual sensory system is non-functional in individuals with congenital blindness. Therefore, understanding the functioning of the vestibular system is pertinent. Individuals with low vision or congenital blindness may also be prone to vestibular disorders and, hence, may need to be evaluated using a vestibular battery of tests.
Provided certain guidelines to conduct perioperative vestibular assessment and testing5. The patient to be positioned supine on a flat surface, with the head tilted up about 30° and video goggles in place. The heated water temperature should be 44 °C, and the cool water temperature should be 30 °C. Each ear is irrigated with warm and then cool water for 30 seconds per stimulation, for a total of four stimulation trials. A sufficient amount of time is allowed between irrigations to allow for the resolution of subjective vestibular stimulation and nystagmus. Counting numbers, naming games and other mental alerting tasks should be offered during the test. The post-stimulation nystagmus should be recorded until the peak velocity is reached, at which point visual fixation can be added. The UW (unilateral weakness) score is also known as the reduced vestibular response score and is the most useful variable, as it is highly sensitive and specific for detecting a unilateral peripheral vestibular loss, though the lesion could be at any site between the horizontal semi-circular canal and the root entry zone of the eighth cranial nerve at the cerebellopontine angle. Most centers use a normal UW score cut-off of 20%-25%. A large variety of vestibular disorders can cause an abnormal UW score, including MD, meningitis, vestibular schwannoma, and vestibular neuritis. The DP score is much more controversial, and its role in the surgical vestibular patient is negligible. It is most commonly abnormal because of spontaneous nystagmus. An abnormal FI indicates cerebellar dysfunction, with the normal cut-off ranging between testing centers from 60% to 70%.
Studied Brazilian population of 40 individuals and compared air and water caloric measures [24]. They subjected them to a neurotological evaluation, which included caloric testing with air at 50°C and 24°C and water at 44°C and 30°C. They concluded on comparison between 50°C and 24°C air caloric tests, and 44°C and 30°C water caloric tests, that similar slow-phase velocity values for both ears, higher responses in the cold temperature and in the water test were noted, and similar results of unilateral weakness or directional preponderance for post-caloric nystagmus in both tests. Carried out a clinical comparative study of caloric tests with water or air in healthy individuals and a diagnosis study on air caloric tests on the Chinese population, and they concluded that air caloric tests can be used instead of water caloric tests in clinics [25]. And if the patient has no contraindications, an air caloric test can be used as a priority. Studied on 91 subjects , air and water caloric stimulation with water at 44°C and 30°C and air at 50°C and 24°C, to conclude that the stimulus with air is similar to stimulation with water, including absolute values [26].
A major drawback with the caloric test is the implicit presumption that the absolute responses to caloric irrigations have a normal distribution and uneven variation. This is demonstrated by the fact that the majority of laboratories would employ a predetermined percentage difference (often 20–30%) as a standard for an abnormal difference between the responsiveness in the patient's right and left ears. When dealing with slow-component velocity responses of 15°/s, one can achieve the criteria by using fixed criteria and percentage differences with very little difference between the ears for absolute values; however, if working with responses that are > 40°/s, much larger differences in the absolute values would be needed to achieve the same percentage difference criteria.
Evaluated air caloric weakness and VEMP latency and amplitude among 37 individuals with simple chronic otitis media and conductive hearing loss owing to unilateral COM without cholesteatoma [27]. They observed that results from either the caloric test or the VEMP test revealed a high incidence rate (81.1%) of abnormalities in patients with unilateral uncomplicated CSOM and recommended preoperative vestibular function be integrated in testing patients with CSOM.
Studied electronystagmography analysis of caloric test parameters in vestibular disorders [28]. Among the normal vestibular system group, the average caloric nystagmus SPV was 17.4°/s, while the average Slow Phase Velocities (SPV) in the peripheral lesion groups were reduced on the affected side, as expected. On the unaffected side of a compensated vestibular lesion, the average SPV of caloric nystagmus was also reduced, which could be due to the effect of the central adaptive systems. According to their findings, central dysfunctions increase both the average caloric average SPV and the spontaneous nystagmus average SPV (25.0°/s), showing that in central vestibular injuries, the central inhibitory mechanisms of the caloric response are disrupted. Their results signified the importance of electronystagmography analysis of spontaneous and caloric nystagmus in the evaluation of dizzy patients.
Studied patterns of vestibular dysfunction in chronic Traumatic Brain Injury (TBI) among ninety nine individuals of 15-49 years range [29]. In 33 of 99 patients (33.3%), abnormalities involving one or more components of the vestibular labyrinth and/or nerve divisions were discovered. The horizontal semi-circular canal was the most commonly affected (18.2%), followed by the saccule (14.1%), utricle (8.1%), posterior (7.1%), and anterior (2.0%). Vestibular dysfunction was linked to skull-base fractures, superior canal dehiscence, and localized ear trauma. They concluded that chronic TBI was related to reduced postural stability for tasks requiring a high level of vestibular and visual input for balance. Vestibular hypofunction was detected using vHIT, VEMP, and caloric tests.
Looked at 57 patients with Meniere’s disease (MD) with significant unilateral canal paresis (> 25%) 61.4%of patients, having reduced caloric responses as compared to the non-MD ear [30]. The remaining 38.6% of patients had normal symmetric responses.
Diagnosing the cause of episodic spontaneous vertigo is a critical task that will likely provide symptom relief to many vertigo sufferers. Their findings suggested that combining audiometry, vHIT, VEMP, caloric test, and ictal nystagmus event monitoring may yield better diagnostic separation than each test alone. The creation of globally standardized vestibular test parameters and a case history questionnaire should be an aim of the neuro-otology and audiology specialties in order to improve the yield and accuracy of diagnostic criteria for Meniere’s illness. Some subjective screening tests like Tandem gait test, Romberg test, Fukuda stepping test, Gait test were used. Figure nose test to screen the participants of congenital blindness individuals to perform an air caloric test.
Conducted objective vestibular tests on 120 people (Indian origin) between the ages of 18 and 55 [31]. All of the right-handed individuals with no significant audio-vestibular dysfunctional history were included, where as those who reported neck discomfort, a history of neurological disease, drunkenness, or training in martial arts, yoga, dancing, or gymnastics were excluded from the study. The unilateral weakness (UW) and Directional Preponderance (DP) on the caloric test were 17.00/sec ± 13.70/sec and 15.00/sec ± 110/sec respectively. Bi-thermal air stimulation (50°C and 24°C) was used in this study. The test revealed symmetrical reactions between the ears, which corresponded with the findings of previous studies. As the values can vary depending on the instrumentation used and other variables, each laboratory is recommended to have its own norms (BSA, 2006). Therefore, the present study was undertaken.
Caloric Investigations in Blind Individuals and low vision individuals
Conducted a systematic on balance in the blind regarding issues like balance adaptation in the blind and the effects of training regimens on blind balance12. They concluded that the blind have poor balance, and did not differ from normal people when enough data from the vestibular and proprioception systems is provided. Balance improves with age among blind, increasing the efficiency and maturity of the vestibular and proprioception systems.
Lack of vision degrades an individual's performance balance control when compared to their sighted peers [32]. People who are sighted use their ankle joints more to overcome external postural disruption and maintain or recover their balance, whereas people who are blind use their hip joints more12. When blind people face a sensory disturbance or an acute perturbation, they usually rely on somatosensory information as a preferred and superior balance system for better regulation of body posture and balance recovery.
Studied carried postural stability among seventy (20-40 years) people with visual impairment in the Saudi Arabian population1. COG velocity while standing on an unstable surface was measured and they reported regardless of eye open or closed condition, they behave in the same manner as sighted subjects with eyes closed.
Visually impaired individuals have impairments in both dynamic and static balance as compared to individuals with sight and the balance improved with age in persons with visual impairment, although balance regulation was dependent on the proprioception and vestibular systems. Individuals with sight had superior balance even than those with visual impairment into sports [33].
On comparison of vestibular reactivity among individuals with congenital idiopathic nystagmus and blindness on caloric test, it was reported that vertigo in congenital nystagmus individuals was much lower than in normal sight individuals. Also, congenital nystagmus with no vestibulo-ocular response gets less dizzy after vestibular stimulation than normal.
The air caloric is reported to be more effective and easier to administration for all age groups of populations compared to water caloric, which is the gold standard for vestibular testing. Duration of irrigation is a factor that influences the results of the air caloric testing, and the temperature of the testing may need to be different than that of the water caloric, considering air is a poor conductor of temperature.
Therefore, there is a need to establish standard testing protocols that can help in cases of normal vision and individuals with congenital blindness. The aim of the study was to conduct a preliminary exploratory study to evaluate responses to air caloric using videonystagmography among neurotypical adults and individuals with congenital blindness.
Methods
Participants:
Two groups of individuals within 18-45 years were recruited for the study. One group of Neurotypical adults and other group of individuals with congenital blindness. The study was conducted during 2022-23. The individuals with congenital blindness were recruited with their consent from the regional centre for disabled. They were oriented about the procedure, the need for the study, and safety issues, before administration of the tests. Participants with normal otoscopic examination, pure tone thresholds within normal limits and immittance audiometry, with present acoustic reflexes were recruited for both the groups. All the individuals were screened for any known ear pathologies, history and complaint of any vestibular symptoms, ear surgeries, or history of alcohol consumption within last 24 hrs of the evaluations.
Equipment used:
a. BalanceEye VOG from cyclops (2022) with infrared goggles (2 camera system) was used to record responses.
b. KALORICstar air irrigator was used to stimulate ears in cold and warm conditions. Model number: BioMed 12/1-2022.594
Procedure:
Participants were instructed about the procedure of the evaluation and were informed about avoiding heavy food in the last two hours of test time, avoidance of alcohol, coffee in the last 24 hours, avoiding makeup to face, eye, etc.
Flow chart of the procedure:
Screening for audiological and vestibular issues: Basic audiological test battery was carried out including otoscopic evaluation, pure tone audiometry, tympanometry, Dizziness Handicap Inventory administration and subjective Vestibular Assessment.
Subjective vestibular tests: Fukuda Stepping Test, Romberg’s test, sharpened Romberg test, Tandem gait, Finger to nose test, walking balance through variations on tandem walking, were administered and the results were recorded.
Air caloric test: The test was conducted in a semi dark room with patient was seated comfortably on an automated ENT chair tilted to a supine position with the head rest lifted to 30 degrees. The calibration was carried out prior to caloric testing with the patient seated in front of a TV screen and stimulus projected onto the screen by the cyclops software. Nystagmus are monitored using the VNG system (BalanceEye VOG from Cyclops). The recording was manually inspected for the slow phase velocity (SPV) and direction of the nystagmus, which were recorded for data analysis. The test sequence was carried out in order of right warm, left cold, left warm, and right cold. The inter task duration was 5 minutes. The test was carried out in two sessions, kept apart for a week to 10 days. The first session was conducted with a 60 sec of irrigation duration, and the second session was conducted with a 75 sec irrigation duration. Temperature was maintained at 24°C for cold air caloric and 50°C/ 48°C for warm air caloric for duration of 60 seconds and 75seconds. Recording of the response coincided with irrigation onset and lasted for 2 minutes after the end of irrigation. At the end of the 1-minute post start of irrigation, the visual fixation suppression protocol was applied.
And all the individuals with congenital blindness (partial or complete) were sub-grouped into two groups, group A consisting of individuals with blindness individuals, and who passed the subjective vestibular screening. While group B consisted of individuals with blindness individuals who failed subjective vestibular screening.
Individual descriptive statistics were applied (directional preponderance and linear paresis are calculated by using Jonkeeys formula) while group comparison statistics were applied to compare SPV values of air caloric stimulation of 75s versus 60s duration and comparison of SPV values on neurotypical adults and blindness individuals.
Results and Discussion
Though thirty one participants were initially recruited for the neurotypical adults group, based on the criteria nine of them were excluded due to motion sickness. Few of them even showed unwillingness to participate in the second 60s stimulation of air caloric. For the group of individuals with congenital blindness, fifteen participants were recruited among which, ten participants were excluded due to not being able to record pupil movement on the Balance eye VNG system. They were found to have cataracts and/or were unable to open their eyes completely. Therefore, the system could not identify the eyelid or pupil, and recording was not possible. Hence the group consisted of only five subjects. They could complete the full test of caloric for 75sec stimulation condition. All of them found the experience to be new and tolerable. But we were unwilling for another test with a 60 sec duration. Therefore, testing was stopped after one recording of all four tasks (RW, RC, LW, and LC) for 75 seconds (Table 1).
| Participants | No. of participants | Age Range |
|---|---|---|
| Neurotypical group | 22 | 18-45 |
| Congenitally blind group | 5 | 18-45 |
Table 1: Demographic data of the participants taken for analysis.
Air caloric using videonystagmography among neurotypical adults:
The table 2 shows that mean SPV values varied for both 60 seconds and 75 seconds duration for all caloric conditions (RW, RC, LW, LC), but were higher for 75s stimulation duration than 60s stimulation duration. It was observed that out of 22 participants, thirteen participants had differences in SPV of RW across the stimulation of 75s and 60s of air caloric, ten participants had differences in SPV LW across the stimulation of 75s and 60s of air caloric, thirteen had differences in SPV RC across the stimulation of 75s and 60s of air caloric, eleven had differences in SPV LC across the stimulation of 75s and 60s of air caloric (Table 2).
| Parameters | Stimulus duration of 75s | Stimulus duration of 60s | ||
|---|---|---|---|---|
| Mean ± SD | Range (Min – Max) | (Mean ± SD) | Range (Min – Max) | |
| Right ear Warm -RW | 16.622 ± 6.057 | 4.92 - 28.45 | 11.355+/-7.201 | .00 - 26.14 |
| Right ear Cold-RC | 19.304 ± 7.549 | 7.69 - 40.33 | 16.975+/-8.268 | 4.62 - 36.06 |
| Left ear Warm-LW | 17.508 ± 8.386 | 4.94 - 36.35 | 18.042+/-8.628 | 5.78 - 46.34 |
| Left ear Cold-LC | 20.435 ± 5.734 | 11.90 - 31.38 | 16.058+/-6.277 | 4.62 - 35.65 |
| unilateral Weakness | 22.0232 ± 7.217 | .70 - 118.56 | 63.4121+/-7.762 | 1.58 - 645.07 |
| Right Beating | 37.027 ± 9.891 | 21.59 - 56.79 | 26.876+/-9.831 | 6.78 - 41.29 |
| Left Beating | 33.107 ± 12.388 | 6.50 - 55.94 | 33.035+/-2.317 | 10.40 - 52.96 |
| Directional Preponderance | 16.726 ± 4.310 | .53 - 53.71 | 52.433117.324 | 1.85 - 500.14 |
| Gain Asymmetry | 31.825 ± 4.538 | .53 - 292.05 | 51.1141+/-7.702 | .21 - 500.14 |
| Total RE Response | 32.14 ± 3.056 | 7.04 - 58.72 | 27.618+/-2.397 | 4.62 - 49.71 |
| Total LE Response | 34.749 ± 4.053 | 5.02 - 65.18 | 33.48+/-9.767 | 12.57 - 46.66 |
| Total Response | 66.432 ± 9.520 | 28.25 - 99.98 | 56.020+/-3.443 | 12.52 - 93.70 |
Table 2: Caloric responses (SPV) for neurotypical adults group.
A test of normality was conducted to check if the data meets normality assumptions, and the results indicated that the data satisfied assumptions of normality; hence, a parametric test such as an independent t test was performed to assess if there is any significant difference in the parameter scores across the groups (Table 3).
| Parameters | Stimulus duration of 75s | Stimulus duration of 60s | t | df | Sig. (2-tailed) | 95%confidence interval | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Right ear Warm -RW | 16.62+/-6.05 | 11.35+/-7.20 | 2.62 | 42 | 0.01 | 1.21 | 9.31 |
| Right ear Cold- RC | 17.50+/-8.38 | 18.04+/-8.62 | -0.21 | 42 | 0.83 | -5.71 | 4.63 |
| Left ear Warm-LW | 19.30+/-7.54 | 16.97+/-8.26 | 0.97 | 42 | 0.33 | -2.48 | 7.14 |
| Left ear Cold-LC | 20.43+/-5.73 | 16.05+/-6.27 | 2.41 | 42 | 2 | 0.71 | 8.03 |
| Unilateral Weakness | 37.02+/-9.89 | 26.87+/-9.83 | 3.41 | 42 | 0.001. | 4.15 | 16.15 |
| Right Beating | 33.10+/-12.38 | 33.03+/-12.31 | 0.01 | 42 | 0.98 | -7.44 | 7.58 |
| Left Beating | 16.721+/-4.31 | 52.431+/-17.32 | -1.41 | 42 | 0.16 | -86.55 | 15.14 |
| Directional Preponderance | 31.826+/-4.53 | 51.1111+/-7.70 | -0.67 | 42 | 0.5 | -77.04 | 38.46 |
| Gain Asymmetry | 32.141+/-3.05 | 27.61+/-12.39 | 1.17 | 42 | 0.24 | -3.22 | 12.26 |
| Total RE Response | 34.741+/-4.0538 | 33.48+/-9.76 | 0.34 | 42 | 0.73 | -6.09 | 8.62 |
| Total LE Response | 66.431+/-9.52 | 56.022+/-3.44 | 1.6 | 42 | 0.11 | -2.71 | 23.53 |
| Total Response | 22.022+/-7.21 | 63.411+/-7.76 | -1.29 | 42 | 0.2 | -106.03 | 23.25 |
Table 3: Comparison of caloric test results between stimulus duration of 75s and 60s in neurotypical adults.
The independent t test was performed to find the significant difference in each of the parameters between stimulation duration 75s and stimulation duration 60sec. From the results it was found that for RW, LC, and unilateral weakness there was significant differences in RW, RIGHT BEATING, and LEFT BEATING for stimulation of air caloric (Table 4).
| Stimulus Duration | Stimuli | Right ear (mean sd) | Left ear (mean sd) | t-test statistic value | df | p-value | 95% of confidence interval | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| Stimulus duration of 75s | WARM | 16.62 ± 6.05 | 17.50 ± 8.38 | 0.39 | 42 | 0.692 | -5.33 | 3.57 |
| (4.92– 28.45) | (4.94 – 36.35) | |||||||
| COLD | 19.30 ± 7.54 | 20.43 ± 5.73 | 0.56 | 42 | 0.578 | -5.34 | 3.58 | |
| (7.69 – 40.33) | (11.9 – 31.38) | |||||||
| Stimulus duration of 60s | WARM | 11.35 ± 7.20 | 18.04 ± 8.62 | 2.79 | 42 | 0.008 | -5.21 | 2.94 |
| (0 – 26.14) | (5.78 – 46.34) | |||||||
| COLD | 16.97 ± 8.26 | 16.05 ± 6.27 | 0.41 | 42 | 0.681 | -5.21 | 2.95 | |
| (4.62 – 36.06) | (4.62 – 36.06) | |||||||
Table 4: Results of independent t test for left and right ear comparison.
The effect of ear on caloric test:
Shapiro wilk test for normality was carried out to check if the data meets the normality assumption. The results revealed that the data satisfies the normality assumption (p > 0.05) hence parametric test was conducted to assess the significant difference in mean scores across the groups.
Independent t test was conducted to assess the significant difference in the mean scores of Warm and Cold conditions across left and right ear, and the result indicate that there is a statistically significant difference in Warm condition of stimulus duration 60s across the groups (t (42) = -2.791, p = 0.008). but no significant difference was observed in Warm and Cold conditions of stimulus duration of 75s and Cold condition of stimulus duration of 60s across the groups (Table 5).
| Parameters | Neurotypical Group (mean sd) | Congenital Blindness (mean sd) | t | df | Sig. (2-tailed) | 95% of confidence interval | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| RW | 16.62 ± 6.05 | 13.90 ± 12.35 | 0.767 | 26 | 0.45 | -4.55 | 9.98 |
| LW | 17.50 ± 8.38 | 14.75 ± 11.50 | 0.658 | 26 | 0.517 | -5.84 | 11.33 |
| RC | 19.30 ± 7.54 | 19.99 ± 13.91 | -0.164 | 26 | 0.871 | -9.32 | 7.95 |
| LC | 20.43 ± 5.73 | 14.61 ± 11.92 | 1.722 | 26 | 0.097 | -1.12 | 12.77 |
Table 5: Results of independent t test for comparison between neurotypical and individuals with congenital blindness group.
The effect of standard air caloric protocol (75s) on individuals with normal hearing and congenital blindness:
The mean values in SPV of the neurotypical individual’s group for RW was 16.620/sec+/-6.050/sec while for individuals with congenital blindness group was 13.900/sec+/-12.350/sec. Similarly, for LW, RC and LC conditions the SPV values were 17.500/sec+/-8.380/sec, 19.300/sec+/-13.910/sec, 20.430/sec+/-5.730/sec for the neurotypical individuals group. For individuals with congenital blindness group, the SPV values for LW, RC and LC are 14.750/sec+/-11.500/sec, 19.990/sec+/-13.910/sec and 14.610/sec+/-11.920/sec respectively. The independent t test revealed no significant difference on RW, LW, RC & LC between the neurotypical group and individuals with congenital blindness. (p > 0.05). The outcome of the study aimed to compare SPV values for cold irrigation of 75s duration and 60s duration among neurotypical young adults. The result revealed that there was a significant difference for the measured SPV.
The study findings showed that the duration of stimulation has an effect on the obtained SPV values among neurotypical adults. However, the values were within the normative range of other authors. Out of 22 participants who completed both the testing, 13 of participants were showing difference in RW, 10 of participants were showing difference in LW, 13 of participants were having significant difference in RC and 11 of participants were having significant difference in LC. The values were higher for 75s, and the duration was well tolerated by all participants. Therefore, it is recommended for routine clinical use.
The obtained mean value and standard deviation for parameters during 75 s of stimulation of air caloric among the neurotypical adults RW, LW, RC, and LC were 16.620/sec ± 6.05, 17.500/sec ± 8.38, 19.300/sec ± 7.54, and 20.430/sec ± 5.73 respectively. The results of the study by [34] show air caloric values to be RC 15.90/sec ± 9.80/sec, LC 16.60/sec ± 10.70/sec, RW 17.90/sec ± 14.70/sec, LW 19.30/sec ± 16.30/sec, and total SPV values for RE 33.70/sec ± 22.40/sec and LE 35.90/sec ± 24.70/sec, respectively, which is similar to values obtained by our study. The obtained mean value and standard deviation of parameters during 60s stimulation of air caloric among neurotypical adults RW, LW, RC, and LC were 11.350/sec ± 7.200/sec, 18.040/sec ± 8.620/sec, 16.97 ± 8.260/sec, and 16.050/sec ± 6.270/sec, respectively.
As observed, SPV values are higher during the 75s stimulation of air caloric than the 60s for individuals with neurotypical adults. Hence, 75s stimulation of air caloric teat was performed for individuals with low vision blindness first. Of the five individuals who underwent the testing, none returned for the second visit for 60s duration testing.
Some of the difficulties noted for testing for congenital blindness were the presence of cataracts; 06 of 15 individuals had cataracts that totally covered their pupils, and then detection of the pupils by an infrared camera was not possible, unable to open eyelid fully, therefore inability to record the pupil by the infrared camera and co-occurring vestibular disorders, as evidenced by failure in subjective vestibular testing. VNG testing for caloric is challenging for individuals with congenital blindness. Probably further studies will explore ENG for the same purposes.
The obtained mean value and standard deviation for parameters during 75s stimulation of air caloric among the neurotypical adults RW, LW, RC, and LC were 16.620/sec ± 6.050/sec, 17.500/sec ± 8.380/sec, 19.300/sec ± 7.540/sec0/sec, and 20.430/sec ± 5.730/sec, respectively. The obtained mean value and standard deviation of parameters during 60s stimulation of air caloric among neurotypical adults RW, LW, RC, and LC were 11.350/sec ± 7.200/sec, 18.040/sec ± 8.620/sec, 16.970/sec ± 8.260/sec, and 16.050/sec ± 6.270/sec, respectively.
The present study explored the effect of the ear on air caloric and compared SPV values for warm and cold irrigation of 75s and 60s stimulation among neurotypical young adults. The results revealed that there is a significant difference in the mean scores of warm and cold conditions across the left and right ear, and the results indicate that there is a statistically significant difference in warm condition of stimulus duration 60s across the groups t (42) = -2.791, p = 0.008). However, there was no significant difference between the groups in the warm and cold conditions of stimulus durations of 75s and 60s.
Carried out a study on the effects of nystagmus on the air and water caloric tests on the Brazilian population [35]. The objective of the study is to compare the nystagmus response in the caloric tests with air at 50ºC and 24ºC and with water at 44ºC and 30ºC. The study group was made up of 40 volunteers, 25 females and 15 males, with ages between 18 and 40 years, without ear complaints. The results of the study found that cold stimulation elicited a higher SPV than warm stimulation. This is in contradiction to the findings of the present study.
Carried out a study in 2021 on Caloric Stimulation with Water and Air: Responses by Age and Gender caloric Bithermal Stimulation with water at temperatures of 44°C and 30°C (Micromedical Technologies, Inc., USA) and air at temperatures of 50°C and 24°C (Micromedical Technologies, Inc., USA) [36]. 91 subjects were evaluated (46 men and 45 women) with a mean age of 43 years old.
Comparing the SPV values obtained in each ear with water and air stimulation, no difference was observed in relation to the stimulation side, which is similar to the findings of the present study. They also compared SPV values with air and water stimulation in relation to age groups >60 years and <60 years. It was observed that the SPV was lower for individuals in the group aged over 60 years compared to the other groups.
The investigate the effect of the standard air caloric protocol on individuals with normal hearing and congenital blindness. To check for any statistically significant differences in the parameters of RW, LW, RC, and LC between the case and control groups. The results revealed that there is no statistical significance between the parameters of RW, LW, RC, and LC between the two groups (p > 0.05). The obtained mean value and standard deviation for parameters during 75 s of stimulation of air caloric among participants with low vision blindness (RW, LW, RC, and LC) were 13.900/sec ± 12.350/sec, 14.750/sec ± 11.500/sec, 19.990/sec ± 13.910/sec and 14.610/sec ± 11.920/sec, respectively.
Ahmad et al., 2019 carried out a study on postural stability in people with visual impairments in the Saudi Arabian population. The findings revealed that the mean COG velocity when standing on a foam surface differed between visually impaired and sighted volunteers with their eyes open and closed. The mean COG velocity in the visually impaired group was substantially higher than in the sighted group with eyes open. The mean COG velocity of the visually challenged group and the sighted group with eyes closed, however, did not differ. Notably, there was a significant difference in mean COG velocity between sighted subjects with eyes open and sighted subjects with eyes closed.
When eyesight is lost, the brain loses around half of its usual sensory information [37]. Blind people have abnormal postural reflexes and motor patterns, which result in an abnormal distribution of muscle forces in the body, resulting in postural and balance deficits [38,39]. Furthermore, "hand-to-eye" synchronization is lost, requiring the hands to undertake both perception and execution duties [40]. Body mechanics are affected by sensory feedback loss, leading to imbalance, a lack of protective reflexes, and motor and neurological disorders [13,14]. The ability of muscles to exert force is unaffected by vision; nonetheless, neuro-sensory motor development in blind people may contribute to balance disruption [10,11]. Such complex interactions may explain the significant reduction in postural stability in participants with visual impairment.
Little is known about the differences in postural control between people with congenital and acquired blindness. Blind people adapt to their disability by establishing postural adjustment mechanisms [7-9] suggests that the timing of the injury and the rehabilitation program can promote compensatory mechanisms for postural regulation. Environmental restrictions have been found in studies to influence the pattern of postural responses [2]. Congenitally blind children have a better ability to combine available nonvisual feedback, such as auditory feedback, and benefit from neuroplasticity of the unused visual cortex, as in Braille reading [5-10]. With extensive use, practice, and training, neural connections can be modified with extensive use, practice, and training [25-31].
Carried out a study on vestibular reactivity in cases of congenital nystagmus and blindness [35]. Twenty cases of congenital idiopathic nystagmus and twenty-one cases of blindness without complicating disabilities were all irrigated with water at 30°C and 44°C in the right and left ear for 40 seconds. The eyes were open. The calorizations were performed in darkness unless the patient was suffering from amaurosis of both eyes. For blindness, the material has been divided into two groups according to the absence or presence of vestibulo-ocular reflexes. Group 1: 12 cases. The group comprised those patients who did not show vestibulo-ocular reflexes on caloric testing. None of the first 09 cases exhibited any vestibulo-ocular reactions to any of the four calorizations. Cases 10, 11, and 12 showed no vestibulo-ocular reactions on some of the irrigations and doubtful ones on the others. Group 2: 09 cases, all showing weak vestibulo-ocular reactions to all calorizations. And they concluded that in congenital nystagmus individuals with absent vestibulo-ocular reactions, the vestibulo-spinal reactions are of normal magnitude and have smaller variations than normal The values for vertigo are significantly lower in congenital nystagmus than in the normal material, according to the z-test, z being 7.24. Cases of congenital nystagmus with no vestibulo-ocular reactivity become less dizzy from vestibular stimulation than normal.
Conclusion
The mean value for parameters measured during 75s of air caloric stimulation in neurotypical individuals RW, LW, RC, and LC was 16.620/sec ± 6.050/sec, 17.500/sec ± 8.380/sec, 19.300/sec ± 7.540/sec, and 20.430/sec ± 5.730/sec respectively. The mean and standard deviation of parameters measured during 60 seconds of air caloric stimulation in neurotypical individuals RW, LW, RC, and LC were 11.350/sec ± 7.200/sec, 18.040/sec ± 8.620/sec, 16.970/sec ± 8.260/sec, and 16.050/sec ± 6.270/sec, respectively. The responses did not differ between the ear, i,.e right ear and left ear results were similar. The responses for warm and cold conditions also did not differ significantly on statistical testing.
Limitations of the Study
The required sample size is 31 participants but 7 participants were excluded due to motion sickness and unwillingness to participate in second phase of testing. Fifteen participants of congenital blindness were tested 6-7 participants were having mild to moderate sensorineural hearing loss and 2 participants were having conductive hearing loss and 1 participant is having mixed hearing loss. Only 5 participants were able to complete air caloric test. For individuals with small opening of eye and cataract individuals where pupil detection was not happening through the goggles could not able to perform air caloric test to assess vestibular system. For individuals with congenital blindness who are all blinking eyes majority of the time could not be able to detect pupil through goggles.
References
- Alghadir AH, Alotaibi AZ, Iqbal ZA. Postural stability in people with visual impairment. Brain Behav. 2019;9(11):e01436.
- Abouzayd M, Smith PF, Moreau S, Hitier M. What vestibular tests to choose in symptomatic patients after a cochlear implant? A systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2017;274:53-63.
- Barin K. Caloric testing. GP Jacobson, NP Shepard, k Barin, K Janky, D McCaslin, Balance Function Assessment and Management. 2020:257-82.
- Bittar RS, Sato ES, Silva-Ribeiro DJ, Oiticica J, Mezzalira R, Tsuji RK, et al. Caloric test and video head impulse test sensitivity as vestibular impairment predictors before cochlear implant surgery. Clinics. 2019;74:e786.
- Brodsky JR, Zhou G. Perioperative vestibular assessment and testing. Oper Tech Otolaryngol Head Neck Surg. 2019;30(3):162-70.
- de Barros AC, Caovilla HH. From nystagmus to the air and water caloric tests. Braz J Otorhinolaryngol. 2012;78(4):120-5.
- Cozma S, Ghiciuc CM, Damian L, Pasquali V, Saponaro A, Lupusoru EC, et al. Distinct activation of the sympathetic adreno-medullar system and hypothalamus pituitary adrenal axis following the caloric vestibular test in healthy subjects. Plos One. 2018;13(3):e0193963.
- Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35(3):185-96.
- Choi JE, Kim YK, Cho YS, Lee K, Park HW, Yoon SH, et al. Morphological correlation between caloric tests and vestibular hydrops in Ménière's disease using intravenous Gd enhanced inner ear MRI. Plos One. 2017;12(11):e0188301.
- Cordero-Yanza JA, Arrieta Vázquez EV, Hernaiz Leonardo JC, Mancera Sánchez J, Hernández Palestina MS, Pérez-Fernández N. Comparative study between the caloric vestibular and the video-head impulse tests in unilateral Menière’s disease. Acta Otolaryngol. 2017;137(11):1178-82.
- Cooper JC, Mason RL. Variability of air calorics vs water: statistical implications. Arch Otolaryngol. 1979;105(3):113-5.
- Daneshmandi H, Norasteh AA, Zarei H. Balance in the blind: A systematic review. J Phys Ther. 2021;11(1):1-2.
- Du Y, Ren L, Liu X, Wu Z. Machine learning method intervention: Determine proper screening tests for vestibular disorders. Auris Nasus Larynx. 2022;49(4):564-70.
- Felipe L, Cavazos R. Caloric stimulation with water and air: responses by age and gender. Iran J Otorhinolaryngol. 2021;33(115):71.
- Forssman B. Vestibular reactivity in cases of congenital nystagmus and blindness. Acta Otolaryngol. 1964;57(3-6):539-55.
- Gonçalves DU, Felipe L, Lima TM. Interpretation and use of caloric testing. Braz J Otorhinolaryngol. 2008;74:440-6.
- Ho KY, Chien CY, Tsai SM, Chen CC, Wang HM. Clinical significance of vestibular function with caloric and vestibular evoked myogenic potential testing for patients with simple chronic otitis media. J Int Adv Otol. 2012;8(3):447.
- Jałocha-Kaczka A, Pietkiewicz P, Zielińska-Bliźniewska H, Miłoński J, Olszewski J. Sensitivity evaluation in air and water caloric stimulation of the vestibular organs using videonystagmography. Otolaryngol Pol. 2014;68(5):227-32.
- Jacobson GP, McCaslin DL, Piker EG, Gruenwald J, Grantham SL, Tegel L. Patterns of abnormality in cVEMP, oVEMP, and caloric tests may provide topological information about vestibular impairment. J Am Acad Audiol. 2011;22(09):601-11.
- Karlsen EA, Mikhail HH, Norris CW, Hassanein RS. Comparison of responses to air, water, and closed-loop caloric irrigators. J Speech Lang Hear Res. 1992;35(1):186-91.
- Kang WS, Lee SH, Yang CJ, Ahn JH, Chung JW, Park HJ. Vestibular function tests for vestibular migraine: clinical implication of video head impulse and caloric tests. Front Neurol. 2016;7:166.
- Mehra YN. Electronystagmography: a study of caloric tests in normal subjects. J Laryngol Otol. 1964;78(5):520-9.
- Mehra YN, Moudgil BD. Evaluation of Ewald's Second Law. An Electronystagmographic Study. Acta Otolaryngol. 1967;63(1):33-41.
- Morrison M, Korda A, Zamaro E, Wagner F, Caversaccio MD, Sauter TC, et al. Paradigm shift in acute dizziness: is caloric testing obsolete?. J Neurol. 2022;269(2):853-60.
- Murphy KA, Anilkumar AC. Caloric testing. 2017.
- Mezzalira R, Bittar RS, do Carmo Bilécki-Stipsky MM, Brugnera C, Grasel SS. Sensitivity of caloric test and video head impulse as screening test for chronic vestibular complaints. Clin. 2017;72(8):469-73.
- Bojrab DI, Lai W, Bojrab DI. Electronystagmography and videonystagmography. J Clin Med. 2019:45-65.
- Mackenzie SW, Iriving R, Monksfield P, Dezso A, Dawe N, Lindley K, et al. Comparing Ocular Responses to Caloric Irrigation and Electrical Vestibular Stimulation in Vestibular Schwannoma. Front Neurol. 2019;10:1181.
- Swamy SN, Yuvaraj P, Pruthi N, Thennarasu K, Rajasekaran AK. Comprehensive normative data for objective vestibular tests. Cureus. 2023;15(6).
- Shepard NT, Jacobson GP. The caloric irrigation test. Handb Clin Neurol. 2016;137:119-31.
- Szirmai A, Keller B. Electronystagmographic analysis of caloric test parameters in vestibular disorders. Eur Arch Otorhinolaryngol. 2013;270:87-91.
- Taylor RL, Wise KJ, Taylor D, Chaudhary S, Thorne PR. Patterns of vestibular dysfunction in chronic traumatic brain injury. Front Neurol. 2022;13:942349.
- Teggi R, Colombo B, Bernasconi L, Bellini C, Comi G, Bussi M. Migrainous vertigo: results of caloric testing and stabilometric findings. J Head Face Pain. 2009;49(3):435-44.
- Weiss N, Tadie JM, Faugeras F, Diehl JL, Fagon JY, Guerot E. Can fast-component of nystagmus on caloric vestibulo-ocular responses predict emergence from vegetative state in ICU?. J Neurol. 2012;259:70-6.
- Winter C, Heath-Kelly C, Kaleem A, Mills C. A moral education? British Values, colour-blindness, and preventing terrorism. Crit Soc Policy. 2022;42(1):85-106.
- Wexler DB, Harker LA, Voots RJ, McCabe BF. Monothermal differential caloric testing in patients with Meniere's disease. Laryngoscope. 1991;101(1):50-5.
- Wen C, Deng Q, Liu Q, Han X, Li S, Chen T, et al. A study of the effect of hot and cold gas perfusion sequence on caloric test results. J Clic Otorhinolaryngol Head Neck Surg. 2021;35(3):209-11.
- Yasumura S, Shojaku H, Watanabe Y. Susceptibility of autonomic symptoms to caloric stimulation in humans. Int J Equilib Res. 2003;62(6):555-62.
- Young AS, Nham B, Bradshaw AP, Calic Z, Pogson JM, Gibson WP, et al. Clinical, oculographic and vestibular test characteristics of Ménière’s disease. J Neurol. 2022:1-8.
- Zarei H, Norasteh AA, Lieberman LJ, Ertel MW, Brian A. Balance control in individuals with visual impairment: a systematic review and meta-analysis. JMCL. 2023;27(4):677-704.