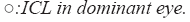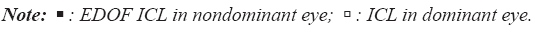Research Article - Journal of Clinical Ophthalmology (2022) Volume 6, Issue 5
A pilot study of visual performance in hybrid monovision technique using an extended depth-of-focus implantable collamer lens (EDOF ICL) in phakic elderly patients
Kimiya Shimizu1,2*, Shuntaro Tsunehiro1,2, Masahide Takahashi1,2, Misae Ito1,2
1 Eye Center, Sanno Hospital, Tokyo, Japan
2 Department of Ophthalmology, International University of Health and Welfare, Tokyo, Japan
- Corresponding Author:
- Kimiya Shimizu
Department of Ophthalmology Shiraz University of Medical Sciences Shiraz Iran
E-mail: mkjoharii@gmail.com
Received: 02-Sep-2022, Manuscript No. AACOVS-22-73479; Editor assigned: 08-Sep-2022, PreQC No. AACOVS-22-73479 (PQ); Reviewed: 22-Sep-2022, QC No. AACOVS-22-73479; Revised: 30-Sep-2022, Manuscript No. AACOVS-22-73479 (R); Published: 07-Oct-2022, DOI: 10.35841/aacovs.6.2.580-586
Citation: Shimizu K, Tsunehiro S, Takahashi M, et al. A pilot study of visual performance in hybrid monovision technique using an Extended Depth-Of-Focus Implantable Collamer Lens (EDOF ICL) in Phakic elderly patients. J Clin Ophthalmol. 2022; 6(5): 580-586
Abstract
Purpose: To assess the clinical results in presbyopic eyes using a hybrid monovision technique in which an Implantable Collamer Lens (ICL) is implanted in the dominant eye and an Extended Depth-Of-Focus (EDOF) ICL is implanted in the nondominant eye.
Subjects and Methods: This prospective pilot study covered 26 eyes of 13 consecutive patients with presbyopia at the Eye Center of Sanno Hospital in Japan. We evaluated the following parameter at 3 months postoperatively: Corrected Distance Visual Acuity (CDVA), DistanceCorrected Intermediate Visual Acuity (DCIVA), Distance-Corrected Near Visual Acuity (DCNVA), Spherical Equivalent (SE), Higher-Order Aberrations (HOAs), Area Under The Log Contrast Sensitivity Function (AULCSF).
Results: A total of 92.3% (12/13 eyes) and 53.9% (7/13 eyes) were corrected to within ± 0.5 D of the target refraction in the dominant eye with an ICL and in the nondominant eye with an EDOF ICL, respectively. The safety index of dominant eye with an ICL was 1.31 ± 0.57 that of nondominant eye with an EDOF ICL was 0.46 ± 0.96. We found significant differences in CDVA (p=0.005), DCNVA (p=0.006), SE (p=0.005), ocular HOAs (p=0.004) and AULCSF (p=0.012) between fellow eyes. The AULCSF of binocular vision with hybrid monovision technique was higher than that of dominant eye implanted with an ICL (p=0.011).
Conclusion: We demonstrated that binocular performance was good in presbyopic patients underwent hybrid monovision technique of ICL surgery, however suggested a need for directly investigating the visual and refractive outcomes and adverse events including HOAs in the eyes with an EDOF ICL.
Keywords
Extended depth-of-focus implantable collamer lens, Hybrid monovision technique, Higher-order aberration, Contrast sensitivity.
Introduction
The EVO Visian Implantable Collamer Lens (ICL, STAAR Surgical, Monrovia, CA, USA) is a posterior chamber phakic intraocular lens that has been used widely for refractive surgery. The V4c (EVO Visian ICL featuring KS-AquaPORT™) model, which has a central hole [1-4], was introduced in 2011 and was designed to facilitate aqueous flow, thus reducing the incidence of cataract and glaucoma compared with the parent model cataracts after surgery. The V5 model (EVO+Visian ICL), which has a larger optical diameter, was introduced in 2016. More recently, the STAAR EVO+ Visian™ ICL with aspheric optic, referred to as the Extended Depth-Of-Focus (EDOF) ICL (STAAR Surgical, Monrovia, California), represents a novel approach to the surgical correction of refractive error and presbyopia in phakic patients and includes a combination of the most advanced elements of the ICL platform, including an increased optic diameter, a central hole (KS-AquaPORT™), and an aspheric design that is theoretically intended to provide up to approximately 2.0 D extended depth-of-focus. Hence, for elderly patients with presbyopia, the options for presbyopic correction are expanding. However, at this time, few reports are available on clinical results of the EDOF ICL [5,6].
On the other hand, the monovision technique is a viable option for the management of presbyopia, in which one eye is corrected for distance vision and the other eye for near vision. The monovision technique by ICL implantation provides good binocular vision at near to far distances, without the development of cataracts, suggested its feasibility as a new surgical approach for early presbyopia [7,8]. Furthermore, in our previous report on presbyopic cataract surgery [9-11], we suggested the technique of hybrid monovision using a multifocal Intra Ocular Lens (IOL). In this method, a monofocal IOL is implanted into the dominant eye and a multifocal IOL into the nondominant eye. The monofocal IOL theoretically ensures excellent distance vision, with a minimal incidence of dysphotopsia, whereas the multifocal lens then provides the patient with relatively good near vision. With regard to the fact that both eyes have the same refraction to distance, the risk of subjective complaints ensuing from anisometropia, as in the case of the monovision technique, is minimized. In addition, the disadvantages of the multifocal IOL (e.g., blurry vision, waxy vision) can be reduced [11]. Therefore, when using an EDOF ICL as this pilot study, we decide to use a presbyopia-correcting technique (i.e., hybrid monovision technique) in which a ICL is implanted into the dominant eye and an EDOF ICL into the contralateral nondominant eye.
The purpose of present study is to prospectively assess the clinical results in presbyopic patients using a hybrid monovision technique for presbyopic ICL surgery.
Materials and Methods
This single-center, prospective study was approved by the institutional review board of Sanno Hospital, Tokyo, Japan (International University of Health and Welfare; 16-S-24) and followed the tenets of the Declaration of Helsinki. Patient data were anonymized before access and/or analysis. All patients provided written informed consent for the surgery after receiving an explanation of the nature and possible consequences of the surgery.
Study population
This study covered 26 eyes of 13 consecutive presbyopic patients (8 men and 5 women) in whom an ICL (ICLV5 or TICLV5) was implanted in the dominant eye determined by hole-in-the-card-test and an EDOF ICL was implanted in the contralateral nondominant eye to correct low to high myopia and myopic astigmatism (manifest Spherical Equivalent (SE) -2.0 diopters (D) or greater). The patients had a mean age of 49.0 ± 4.1 (range, 42-54) years and had subjective symptoms of near visual disturbance when correcting far vision.
The inclusion criteria for this surgical technique were as follows: Corrected Distance Visual Acuity (CDVA) ≥ 20/20, Anterior Chamber Depth (ACD) ≥ 2.8 mm, Endothelial Cell Density (ECD) ≥ 2000 cells/mm2, and no history of ocular surgery, corneal degeneration, cataract, glaucoma, uveitis, or diabetic retinopathy. We excluded keratoconic eyes from this study by using a keratoconus screening test of swept-source anterior segment optical coherent tomography (AS-OCT; CASIA-2, Tomey Corporation, Nagoya, Japan).
Preoperative measurements of axial length used an optical biometer (IOLMaster700; Carl Zeiss Co., Ltd., Kojimachi, Japan). The following determinations were carried out before surgery and 3 months postoperatively: Logarithm of the Minimal Angle of Resolution (logMAR) of Uncorrected Distance Visual Acuity (UDVA) at 5.0 m, CDVA, Distance-Corrected Intermediate Visual Acuity (DCIVA) at 0.7 m, Distance- Corrected Near Visual Acuity (DCNVA) at 0.3 m, manifest refraction, keratometric readings, Higher-Order Aberrations (HOAs), and Contrast Sensitivity (CS), Intra Ocular Pressure (IOP), and ECD, as well as the standard slit-lamp biomicroscopic and funduscopic examinations. IOP was measured using an autokeratometer/autotonometer (TONOREFIII; NIDEK Co., Ltd., Gamagori, Japan). ECD was measured using a noncontact specular microscope (FA-3809IIP, Konan Medical, Inc., Nishinomiya, Japan). We obtained postoperative measurements of the central vault using AS-OCT (CASIA-2). We determined the cornea and ocular HOAs for a 4-mm pupil using the Hartmann–Shack aberrometer (KR-1W, Topcon, Tokyo, Japan). We calculated the root mean square of the third-order (i.e., coma-like aberrations) and fourth-order (i.e., spherical-like aberrations) coefficients separately. CS was measured at four points (3.0 cycles/degrees (cpd), 6.0 cpd, 12.0 cpd, 18.0 cpd) using the CSV-1000™ (Vector Vision, Greenville, SC, USA) at a test distance of 2.5 m. The test was performed with refractive correction. We determined the area under the log CS function (AULCSF) using the CS data, as described previously [12]. Briefly, we plotted the log of CS as a function of log spatial frequency and fitted third-order polynomials to the data. We integrated the fitted function between the fixed limits of log spatial frequencies of 0.48 (corresponding to 3.0 cpd) to 1.26 (corresponding to 18.0 cpd) and determined the obtained value as the area under the log CS function. The patient satisfaction for overall visual performance was assessed at 3 months postoperatively, according to the visual analog scale ranging from 0 (very dissatisfied) to 5 (very satisfied). To understand the cause of dissatisfaction in patients with a score of <3, we conducted a survey on “far, intermediate, and near vision”, “presence of discomfort, asthenopia, and halo and glare.”
ICL power and size calculation
The ICL power was determined with the online calculator of the manufacturer (STAAR Surgical) using a modified vertex formula.
Surgical procedure
The surgical procedures in our institution were comprised as follows, as described previously [13]. In brief, after topical administration of dilating and anesthetic agents, a model V5 ICL was inserted through a 3-mm temporal or superior corneal incision (by the steep meridian of the corneal curvature) with the use of a viscosurgical device into the anterior chamber. The ICL was placed in the posterior chamber, the viscosurgical device was fully washed out with balanced salt solution. Postoperatively, steroidal and antibiotic medications were administered topically 4 times daily for 1 week, and the dose was reduced gradually thereafter. All surgeries were performed by one experienced surgeon (K.S.).
Statistical analysis
Statistical analysis was conducted using commercially available statistical software (Excel-Toukei 2015, Social Survey Research Information Co., Ltd., Tokyo, Japan). The normality of all data samples was first evaluated using the Kolmogorov–Smirnov test. Because the use of parametric statistics was not appropriate, we used the Wilcoxon signed-rank test and the Spearman’s correlation coefficients test. Unless otherwise indicated, the results are expressed as the mean ± standard deviation, and p < 0.05 was considered statistically significant.
Results
Study population
Table 1 summarizes the preoperative patient demographics. No intraoperative complications occurred in the study population, and no eyes were lost to follow up. We found no significant differences in, manifest cylinder (p=0.678), postoperative target refraction (p=0.328), logMAR CDVA (p=0.059), AULCSF (p=0.315), and HOAs (corneal total HOAs, p=0.364; ocular total HOAs, p=0.249) between fellow eyes. For the dominant eyes, we selected nontoric ICL (ICLV5) in 92.3% (12/13 eyes) with the manifest cylinder of 1.25 D or less, or toric ICL (TICLV5) in 7.7% (1/13 eye) with that of 1.5 D, respectively (Table 1).
| Dominant eye implanted ICL | Nondominant eye implanted EDOF ICL | P value | |
|---|---|---|---|
| Manifest spherical equivalent (D) | 5.62 ± 1.95(-2.25 to - 8.50) | -5.27 ± 2.39 - 2.38 to - 9.50) | 0.248 |
| Manifest cylinder (D) | - 0.31 ± 0.48 (0.00 to - 1.50) | -0.40±0.36(0.00 to -1.00) | 0.678 |
| LogMAR UDVA | 1.26 ± 0.24 (0.70 to 1.70) | 1.22 ± 0.25 (0.70 to 1.70) | 0.028 |
| LogMAR CDVA | 0.19 ± 0.10 0.30 to - 0.08) | 0.15 ± 0,09 0.38 to 0.00) | 0.059 |
| LogMAR DCNVA | 0.44 ± 0.25 (0.05 to 0.70) | 0.43 ± 0.24 (0.05 to 0.70) | 0.593 |
| Targeted refraction (D) | 0.10 ± 0.13 0.32 to + 0.16) | 0,00 ± 0.24 -0.31 to + 0.49) | 0.328 |
| Intraocular pressure (mmHg) | 15.1 ± 2.7 (12.0 to 19.0) | 15.0 ± 2.8 (11.0 to 19.0) | 0.779 |
| Axial length (mm) | 26.16 ± 0.87 (25.02 to 27.48) | 26.06 ± 1.01 (24.83 to 27.52) | 0.221 |
| Keratometric values (D) | 43.17 ± 0.73 (42.09 to 44.98) | 43.26 ± 0.80 (42.15 to 45.13) | 0.507 |
| Endothelial cell density (cells/mm²) | 2771 ± 254 (2268 to 3058) | 2749 ± 254 (2299 to 3205) | 0.753 |
| AULCSF | 1.34 ± 0.10 (1.20 to 1.46) | 1.31 ± 0.13 (1.12 to 1.46) | 0.315 |
| Corneal higher-order aberrations | |||
| Total aberrations ( µm) | 0.122 ± 0.050 ( 0.065 to 0.236) | 0.132 ± 0.052 ( 0.084 to 0.273) | 0.364 |
| Coma-like aberrations ( µm ) | 0.105 + 0.057 ( 0.030 to 0.234) | 0.109 ± 0.056 ( 0.063 to 0.248) | 0.861 |
| Spherical-like aberrations (µm) | 0.057 ± 0.019 ( 0.035 to 0.089) | 0.069 ± 0.024 ( 0.034 to 0.113) | 0.101 |
| Ocular higher-order aberrations | |||
| Total HOAs ( µm) | 0.122 ± 0.061 ( 0.050 to 0.298) | 0.130 ± 0.050 ( 0.066 to 0.280) | 0.249 |
| Coma-like aberrations ( µm ) | 0.105 ± 0.067 ( 0.027 to 0.296) | 0.115 ± 0.050 (0.054 to 0.249) | 0.279 |
| Spherical-like aberrations (µm) | 0.051 ± 0.018 (0.029 to 0.059) | 0.057 ± 0.025 (0.025 to 0.128) | 0.311 |
Table 1. Preoperative patient demographics. Note: Data are presented as mean ± standard deviation (range), as applicable. D: Diopter; logMAR: Logarithm of the minimal angle of resolution; UDVA: Uncorrected distance visual acuity; CDVA: Corrected distance visual acuity; DCNVA: Distance corrected near visual acuity; AULCSF: Area under log contrast sensitivity function.
Table 2 summarizes the postoperative demographics. We found no significant differences in, manifest cylinder (p=0.176), logMAR DCIVA (p=0.824), central vault (p=0.311), ECD (p=0.650), but significant differences in manifest SE (p=0.005), logMAR UDVA (p=0.002), logMAR CDVA (p=0.005), logMAR DCNVA (p=0.006), AULCSF (p=0.012), and ocular HOAs (coma-like, p=0.039; spherical-like, p=0.002) between fellow eyes. For the dominant eye implanted with an ICL, the comparison of preoperative and postoperative mean DCNVA was 0.44 (Snellen equivalent=20/50) ± 0.25 and 0.48 (Snellen equivalent=20/60) ± 0.32 logMAR, respectively, and no significant difference was observed (p=0.407). For the nondominant eye implanted with an EDOF ICL, the value was 0.43 (Snellen equivalent=20/50) ± 0.24 and 0.15 (Snellen equivalent=20/27) ± 0.18 logMAR, respectively, and a significant difference was observed (p=0.004) .
| Dominant eye implanted ICL | Nondominant eye implanted EDOF ICL | P value | |
|---|---|---|---|
| Manifest spherical equivalent (D) | -0.15 ± 0.19 0.50 to 0.00) | -0.68 ± 0.39 1.25 to 0.00) | 0.005 |
| Manifest cylinder (D) | 0.08 ± 0.19 (0.00 to - 0.50) | 0.27 ± 0.39 (0.00 to-1.00) | 0.176 |
| Intraocular pressure (mmHg) | 14.5 ± 2.5 (12.0 to 19.0) | 14,4 ± 2.8 (11.0 to 19.0) | 0.799 |
| LogMAR UDVA | -0.14 ± 0.11 -0.30 to 0.00) | 0.16 ± 0.16 (0.00 to 0.52) | 0.002 |
| LogMAR CDVA | 0.21 ± 0,07 -0.30 to - 0.08) | -0.06 ± 0.11 0.30 to 0.10) | 0.005 |
| LogMAR DCIVA | 0.02 ± 0.16 -0.18 to 0.30) | -0.02 ± 0.09 0.08 to 0.10) | 0.824 |
| LogMAR DCNVA | 0.48 ± 0.32 0.08 to 1.00) | 0.15 ± 0.18 -0.08 to 0.52) | 0.006 |
| Central vault ( µm) | 346.8 ± 196.3 (78 to 798) | 369.6 ± 200.1 (91 to 705) | 0.311 |
| Endothelial cell density (cells/mm2) | 2728 ± 225 (2268 to 3086) | 2765 ± 280 (2299 to 3155) | 0.65 |
| Keratometric values (D) | 43.17 ± 0.73 (42.09 to 44.99) | 43.26 ± 0.80 (42.14 to 45.13) | 0.507 |
| AULCSF | 1.32 ± 0.13 (1.20 to 1.61) | 1.18 ± 0.09 (1.11 to 1.27) | 0.012 |
| Corneal higher-order aberrations | |||
| Total aberrations | 0.135 ± 0.056 (0.066 to 0.240) | 0.144 ± 0.056 (0.099 to 0.291) | 0.173 |
| Coma-like aberrations (µm) | 0.114 ± 0.056 (0.038 to 0.238) | 0.127 ± 0.062 (0.052 to 0.282) | 0.152 |
| Spherical-like aberrations (µm) | 0.058 ± 0.025 (0.023 to 0.108) | 0.065 ± 0.017 (0.034 to 0.098) | 0.576 |
| Ocular higher-order aberrations | |||
| Total HOAs (µm) | 0.144 ± 0.073 (0.059 to 0.286) | 0.293 ± 0.068 (0.196 to 0.442) | 0.004 |
| Coma-like aberrations (µm) | 0.128 ± 0.076 (0.052 to 0.283) | 0.220 ± 0.087 (0.096 to 0.412) | 0.039 |
| Spherical-like aberrations (µm) | 0.064 ± 0.039 (0.021 to 0.174) | 0.185 ± 0.025 (0.135 to 0.224) | 0.002 |
Table 2. Postoperative patient demographics. Note: Data are presented as mean ± standard deviation (range), as applicable. D: Diopter; logMAR: Logarithm of the minimal angle of resolution; UDVA: Uncorrected distance visual acuity; CDVA: Corrected distance visual acuity; DCNVA: Distance corrected near visual acuity; AULCSF: Area under log contrast sensitivity function.
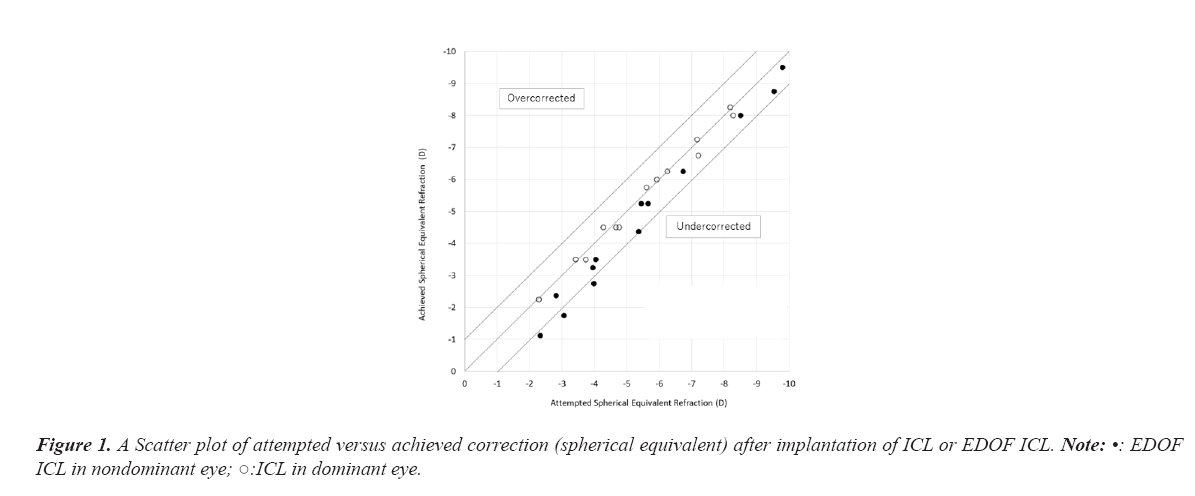
Predictability
Figure 1 depicts a scatter plot of the attempted versus the achieved spherical equivalent correction after implantation of ICL or EDOF ICL. A total of 92.3% (12/13 eyes) and 53.9% (7/13 eyes) were corrected to within ± 0.5 D of the target refraction in the dominant eye with an ICL and in the nondominant eye with an EDOF ICL, respectively. A total of 100% (13/13 eyes) and 76.9% (10/13 eyes) were corrected to within ± 1.0 D of the target refraction in the ICL and in the EDOF ICL, respectively (Figure 1).
Efficacy outcomes
Figure 2 lists the cumulative percentages of eyes attaining specified cumulative levels of UDVA after implantation of ICL or EDOF ICL. The mean UDVA of the dominant eye implanted with an ICL was significantly improved from 1.26 (Snellen equivalent= 20/350) ± 0.24 preoperatively to -0.14 (Snellen equivalent=20/14.5) ± 0.11 logMAR postoperatively (p<0.001). That of the nondominant eye with an EDOF ICL was significantly improved from 1.22 (Snellen equivalent=20/350) ± 0.25 preoperatively to 0.16 (Snellen equivalent=20/27) ± 0.16 logMAR postoperatively (p<0.001) (Figure 2).
Safety outcomes
The postoperative CDVA of 0.0 logMAR (Snellen equivalent=20/20) or better was achieved by 100% (13/13 eyes) of ICL and 84.6% (11/13 eyes) of EDOF ICL, whereas the CDVA of EDOF ICL was lower.
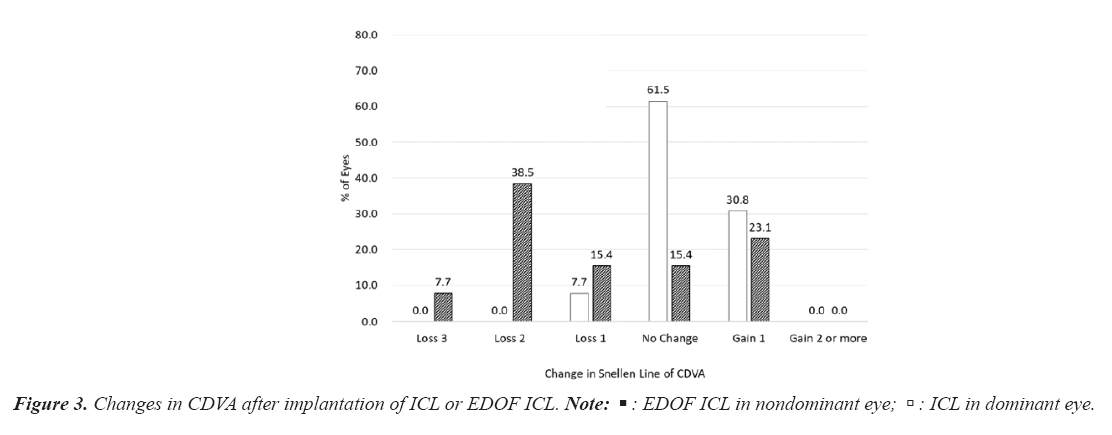
Figure 3 depicts a change in CDVA after implantation of ICL or EDOF ICL. In the dominant eye implanted with an ICL, the mean logMAR CDVA was -0.21 (Snellen equivalent=20/12.5) ± 0.08 (range, -0.30 to -0.08) and the safety index (postoperative CDVA/preoperative CDVA) was 1.31 ± 0.57 (range, 0.58 to 2.22). Eight eyes (61.5%) showed no change in CDVA, four eyes (30.8%) gained one line, and one eye (7.7%) lost one line. In the nondominant eye implanted with an EDOF ICL, the mean logMAR CDVA was -0.06 (Snellen equivalent=20/16) ± 0.10 (range, -0.30 to 0.10), and the safety index was 0.46 ± 0.96 (range, -0.55 to 2.22). Two eyes (15.4%) showed no change in CDVA, three eyes (23.1%) gained one line, two eyes (15.4%) lost one line, five eyes (38.5%) lost two lines, one eye (7.7%) lost three lines (Figure 3).
Contrast sensitivity
Figure 4 displays the comparison of preoperative with postoperative CS. For the dominant eye implanted with an ICL, the comparison of preoperative and postoperative AULCSF was 1.34 ± 0.10 and 1.32 ± 0.13, respectively, and no significant difference was observed (p=0.445). For the nondominant eye implanted with an EDOF ICL, the values were 1.31 ± 0.13 and 1.18 ± 0.09, respectively, and no significant difference was observed (p=0.059). However, the CS of high spatial frequency area was a statistically significant decrease after surgery (12.0 cpd, p=0.033; 18.0 cpd, p=0.044). For binocular vision, the comparison of preoperative and postoperative AULCSF was 1.39 ± 0.08 and 1.38 ± 0.09, respectively, with no significant difference (p=0.128). The postoperative AULCSF of binocular vision with hybrid monovision technique was higher than that of dominant eye implanted with an ICL (p=0.011) (Figure 4).
However, the postoperative mean AULCSF of nondominant eye implanted with an EDOF ICL was lower than that of dominant eye implanted with an ICL (p=0.012; Table 2).
Higher-order-aberration
Preoperatively, the regard to the HOAs for each eye, the comparison of corneal HOAs and ocular HOAs showed no significant differences (dominant eye, p=0.754; nondominant eye, p=0.807, respectively). Postoperatively, for the dominant eye implanted with an ICL, it showed no significant differences (p=0.124), whereas the contrary was significant differences (p=0.002) for the nondominant eye implanted with an EDOF ICL.
For the dominant eye implanted with an ICL, the comparison of preoperative with postoperative ocular HOAs showed no significant differences (coma-like, p=0.221; spherical-like, p=0.149, respectively). For the nondominant eye implanted with an EDOF ICL, a statistically significant increase in ocular HOAs was noted after surgery (coma-like, p=0.006; spherical- like, p<0.001, respectively).
Patient satisfaction
The postoperative satisfaction score in hybrid monovision technique was 3.9 ± 1.0 (range: 2.0 to 5.0). Table 3 summarizes the reason for dissatisfaction in two patients with a score of <3 was lack of visual clarity.
| Questionnaire | Dominant eye implanted ICL eyes (%) | Nondominant eye implanted EDOF ICL eyes (%) | Binocular vision with hybrid monovision patients (%) |
|---|---|---|---|
| Lack of visual clarity | |||
| Far vision | 0 | 2 (15.4) | 0 |
| Intermediate vision | 0 | 1 (7.7) | 0 |
| Near vision | 2 (15.4) | 1 (15.4) | 0 |
| Discomfort | 0 | 2 (15.4) | 0 |
| Asthenopia | 0 | 2 (15.4) | 0 |
| Halo or glare | 0 | 2 (15.4) | 0 |
Table 3. Reasons for dissatisfaction.
Patient 1: A 51-year-old woman had subjective symptoms of near visual disturbance when correcting far vision and was corrected using a hybrid monovision technique. The preoperative CDVA in the nondominant eye was 20/12.5 with sph-5.50 D cyl-0.50 D × ax 110?, and the DCNVA was 20/100. The cycloplegic correction of the nondominant eye preoperatively was sph-5.50 D cyl-0.50 D × ax 110?. The postoperative target refraction was set at sph-0.31 D. At 3 months postoperatively the UDVA was 20/32, the CDVA was 20/25 with sph-0.50 D, and the DCNVA was 20/25. The dominant eye implanted with an ICL showed no change in CDVA, however the nondominant eye with an EDOF ICL lost 3 snellen lines in CDVA. The value of ocular HOAs increased from 0.118 μm to 0.442 μm and AULCSF decreased from 1.44 to 1.04 in the nondominant eye implanted with an EDOF ICL. The satisfaction score was 2.0, which had lack of visual clarity “far, intermediate, and near vision” and stronger discomfort, asthenopia, halo, or glare in the nondominant eye implanted with an EDOF ICL. For binocular vision in hybrid monovision technique, these symptoms disappeared.
Patient 2: A 52-year-old woman had subjective symptoms of near visual disturbance when correcting far vision and was corrected using a hybrid monovision technique. The preoperative CDVA in the nondominant eye was 20/10 with sph-4.25 D, and the DCNVA was 20/100. The cycloplegic correction of the nondominant eye preoperatively was sph-4.25 D. The postoperative target refraction was set at sph-0.21 D. At 3 months postoperatively the UDVA was 20/25, and the CDVA was 20/25 with non corrigunt, and the DCNVA was 20/32. The dominant eye implanted with a ICL showed no change in CDVA, however the nondominant eye with an EDOF ICL lost 2 snellen lines in CDVA. The value of ocular HOAs increased from 0.156 μm to 0.299 μm and AULCSF decreased from 1.34 to 1.20 in the nondominant eye implanted with an EDOF ICL. The satisfaction score was 2.0, which had lack of visual clarity “far vision” and strong discomfort, asthenopia, halo, or glare in the nondominant eye implanted with an EDOF ICL. For binocular vision in hybrid monovision technique, these symptoms disappeared.
Discussion
In this pilot study, we demonstrated that binocular visual performance was good even in presbyopic patients underwent hybrid monovision technique of ICL surgery, however, in nondominant eye implanted with an EDOF ICL, predictability, efficacy and safety index was lower, and the ocular HOAs was increased after surgery.
We also confirmed that the clinical results of ICL were overall good in terms of predictability, efficacy, and safety for correcting low to high ametropia. This finding is in line with previous studies on ICL implantation [1-4] . Until now, only one study has been published on EDOF ICL implantation, and Packer et al first reported that EDOF ICL provides correction of myopia and presbyopia without compromising the quality of vision in patients who desire vision over a continuous range for improved uncorrected near, intermediate and distance visual acuity [6]. In this study population, a significant improvement of DCNVA in nondominant eye implanted with an EDOF ICL was shown (Tables 1 and 2). However, the postoperative UDVA of 0.0 logMAR (Snellen equivalent=20/20) or better was achieved by 100% (13/13 eyes) of ICL and 23.1% (3/13 eyes) of EDOF ICL (Figure 2), whereas the UDVA of EDOF ICL was lower. If this is due to the tendency of postoperative myopia in EDOF ICL, it may be necessary to adjust the refraction targeting. The postoperative CDVA of 0.0 logMAR (Snellen equivalent=20/20) or better was achieved by 100% (13/13 eyes) of ICL and 84.6% (11/13 eyes) of EDOF ICL, whereas the CDVA of EDOF ICL was lower. In addition, we demonstrated that the CDVA decreased in 61.5% (8/13 eyes) of nondomiannt eye with EDOF ICL correction (Figure 3). In the present study, we measured preoperative CDVA with spectacle correction. Retinal magnification with spectacle correction is smaller than that with ICL correction, especially in highly myopic eyes, because the location of the ICL is closer to a nodal point. Accordingly, we know that the improvement of CDVA may be partly explained by reduced magnification of the retinal image in the study population. However, EDOF ICL might induce a large amount of HOAs (Tables 1 and 2) and decrease CDVA (Figure 3) or CS function (Figure 4).
Based on previous studies [14-16], the amount of the ocular HOAs (excluding defocus and astigmatism) increases more than threefold within the age range (20 to 70 years) considered which is unavoidable for everyone. In younger subjects, the internal ocular surfaces (i.e., crystalline lens) compensate, at least in part, for the aberrations associated with the cornea, but this compensation is not present in older subjects. These results suggest that the degradation of ocular optics with age can be explained largely by the loss of the balance between the aberrations of the corneal and the internal surfaces. In our research, there was no evidence of this compensation in 61.5% (8/13 eyes) of nondominant eye implanted with an EDOF ICL (Figure 3), which might be the similar conditions as the degradation of optics with age. This was also considered to be one of the causes of the CS decrease in the high spatial frequency area. However, because the relationship between EDOF ICL and HOAs is not yet clear, we have to investigate with a larger number of patients.
In terms of binocular visual performance in daily life, no patient complained of glare, halo, or discomfort. Therefore, when presbyopic patients with low to high ametropia wish to undergo EDOF ICL surgery, hybrid monovision technique [9-11], in which an ICL is implanted in the dominant eye and an EDOF ICL is implanted in the nondominant eye, might be the best strategy at this time.
One limitation of the present study is that, because this was a pilot study for exploring the clinical results of this new presbyopic approach, the sample size was small with only a 3-month follow-up period. Accordingly, we await further investigations with a larger number of patients and long-term results of hybrid monovision technique or EDOF ICL as well as other complications in this study population.
Conclusion
In conclusion, to the best of our knowledge, this is the first study to assess the hybrid monovision technique using an EDOF ICL in patients with presbyopia. To minimize the impact of the increase in aberration and decrease in CS caused by EDOF ICL, hybrid monovision technique might be the current best strategy; however it is essential to directly investigate the visual and refractive outcomes and adverse events including HOAs in the eyes.
References
- Shimizu K, Kamiya K, Igarashi A, et al. Early clinical outcomes of implantation of posterior chamber phakic intraocular lens with a central hole (Hole ICL) for moderate to high myopia. Br J Ophthalmol. 2012;96(3):409-12.
[Cross Ref] [Google Scholar] [PubMed]
- Shimizu K, Kamiya K, Igarashi A, et al. Long-term comparison of posterior chamber phakic intraocular lens with and without a central hole (hole ICL and conventional ICL) implantation for moderate to high myopia and myopic astigmatism: consort-compliant article. Medicine. 2016;95(14).
[Cross Ref] [Google Scholar] [PubMed]
- Kamiya K, Shimizu K, Igarashi A, et al. Posterior chamber phakic intraocular lens implantation: comparative, multicentre study in 351 eyes with low-to-moderate or high myopia. Br J Ophthalmol. 2018; 102: 177-181.
[Cross Ref] [Google Scholar] [PubMed]
- Shimizu K, Kamiya K, Igarashi A, et al. Intraindividual comparison of visual performance after posterior chamber phakic intraocular lens with and without a central hole implantation for moderate to high myopia. Am J Ophthalmol. 2012;154: 486-494.
[Cross Ref] [Google Scholar] [PubMed]
- Pinto, Hoge. United States Patent Application Publication. 2016.
- Packer M, Alfonso JF, Aramberri J, et al. Performance and Safety of the Extended Depth of Focus Implantable Collamer® Lens (EDOF ICL) in Phakic Subjects with Presbyopia. Clin Ophthalmol. 2020;14: 2717-2730.
[Cross Ref] [Google Scholar] [PubMed]
- Kamiya K, Takahashi M, Takahashi N, et al. Monovision by implantation of posterior chamber phakic intraocular lens with a central hole (Hole ICL) for early presbyopia. Sci Rep. 2017;7: 11302.
[Cross Ref] [Google Scholar] [PubMed]
- Takahashi M, Kamiya K, Shoji N, et al. Intentional undercorrection by implantation of posterior chamber phakic intraocular lens with a central hole (hole ICL) for early presbyopia. BioMed Res Int. 2018;2018.
[Cross Ref] [Google Scholar] [PubMed]
- Iida Y, Shimizu K, Ito M. Pseudophakic monovision using monofocal and multifocal intraocular lenses: hybrid monovision. J Cataract Refract Surg. 2011;37: 2001-2005.
[Cross Ref] [Google Scholar] [PubMed]
- Shimizu K. Monovision strategy. CRST Euro. 2011;50–51.
- Shimizu K, Ito M. Dissatisfaction after bilateral multifocal intraocular lens implantation: an electrophysiology study. J Refract Surg. 2011;27(4):309-12.
[Cross Ref] [Google Scholar] [PubMed]
- Applegate RA, Howland HC, Sharp RP, et al. Corneal aberrations and visual performance after radial keratotomy. J. Refract. Surg. 1998;14: 397–407.
[Cross Ref] [Google Scholar] [PubMed]
- Kato S, Shimizu K, Igarashi A. Assessment of low-vault cases with an implantable collamer lens. PLoS One. 2020;15: e0241814.
[Cross Ref] [Google Scholar] [PubMed]
- Applegate RA. Limits to Vision: Can We Do Better Than Nature?. J Refract Surg. 2000;16: 547–551.
[Cross Ref] [Google Scholar] [PubMed]
- Artal P, Berrio E, Guirao A, et al. Contribution of the cornea and internal surfaces to the change of ocular aberrations with age. JOSA A. 2002;19(1):137-43.
[Cross Ref] [Google Scholar] [PubMed]
- Amano S, Amano Y, Yamagami S, et al. Age-related changes in corneal and ocular higher-order wavefront aberrations. Am J Ophthalmol. 2004;137: 988-992.
[Cross Ref] [Google Scholar] [PubMed]