Case Report - Journal of Primary Care and General Practice (2021) Volume 4, Issue 4
A multi-strain water-based probiotic supplement in the management of irritable bowel syndrome or irritable bowel syndrome-like symptoms and quality of life: A real-world evidence study conducted in the United Kingdom
Simon Rudland1*, Ingvar Bjarnason21Norwich Medical School, Norwich, NR4 7TJ, United Kingdom
2Department of Gastroenterology, King’s College Hospital, London, SE5 9RS, United Kingdom
- *Corresponding Author:
- Simon Rudland
Norwich Medical School,
Norwich, NR4 7TJ, United Kingdom
E-mail: simonrudland@icloud.com
Accepted date: July 15, 2021
Citation: Rudland S, Bjarnason I. A multi-strain Water-based probiotic supplement in the management of irritable bowel syndrome or irritable bowel syndrome-like symptoms and quality of life: A real-world evidence study conducted in the United Kingdom. J Prim Care Gen Pract. 2021;4(4):90–97.
Abstract
Background: Irritable bowel syndrome (IBS) is a common and burdensome functional gastrointestinal disorder of unknown etiology. Due to its complex and multifactorial nature, the delivery of effective management approaches is challenging, with modestly effective therapies and limited efficacy for many conventional drug treatments. To date, some probiotics have shown efficacy in IBS management in randomised clinical trials. The stringent inclusion criteria may not, however, be representative of the wider clinical population. The present study aimed to evaluate the effect of a multi-strain water-based probiotic (Symprove) on participant-reported change in IBS or IBS-like symptoms, and quality of life in a real-life situation. Methods: From 6th February to 31st March 2020, real world evidence data were collected from a total of 2301 individuals who retrospectively completed an online questionnaire, of which 1246 (54%) were trialling for IBS or IBS-like symptoms (medically- or self-diagnosed). P values were calculated using proportions Z tests and Mann-Whitney U tests (non-parametric equivalent). Differences are stated as statistically significant where P ≤ .05. Results: Of the 1246, 58% (n=717) entered the medically-diagnosed pathway and 40% (n=501) entered the self-diagnosed pathway, 2% (n=28) were omitted from the final analysis. Of the final 1218, over 90% of participants in both IBS cohorts reported improvements (‘completely resolved’ or ‘some positive difference’) in IBS or IBS-like symptoms after 4 or more weeks of Symprove, including abdominal pain (p<0.001) bloating (p<0.001), urgency (p<0.001) and bowel habit satisfaction (p<0.001), in addition to quality of life (p<0.001). Conclusions: Trialling Symprove for 4 or more weeks was associated with improvements in IBS or IBS-like symptoms, in addition to quality of life. These results support the effectiveness of Symprove in patients with IBS or IBS-like symptoms and may be considered a potentially useful adjunct for patients in primary and secondary care.
Keywords
Gastrointestinal disorder, Clinical trials, Primary and secondary care, Trialling symprove.
Introduction
Irritable Bowel Syndrome (IBS) is a common functional gastroenterological condition characterised by recurrent abdominal pain, bloating and alterations in stool habits [1]. In addition, many people with IBS present with extraintestinal somatic symptoms. IBS affects approximately 10% of the population [2] and is associated with reduced quality of life (e.g. mental health, relationships), greater impairments in daily activities, in addition to the indirect economic costs on healthcare services (e.g. visits to GPs, prescriptions for medications, investigative procedures) and in the workplace (e.g. increases in absenteeism, lower productivity) [3]. IBS is usually diagnosed in primary care largely based on the Rome IV criteria, of which abdominal pain is a key diagnostic feature [4]. However, in clinical practice there is often overlap in the management of gastrointestinal symptoms for those patients who do not necessarily fulfil the Rome IV criteria for IBS, but still experience IBS-like symptoms. Usually, only the most severe cases of IBS are referred to secondary care for further investigation and management [5].
While the pathophysiology of IBS is unknown, underlying proposed mechanisms include increased visceral hypersensitivity, immune activation, alterations in gut-brain interactions and gut motility. More recently, the gut microbiome has been implicated as a potential driver of IBS pathophysiology [6,7]. It is now well established that this community of microorganisms within the large intestine play a crucial role in regulating gastrointestinal homeostasis and host physiology via complex interactions with the immune system [8]. Revised estimates suggest the human body harbours 38 trillion bacterial cells compared to 30 trillion human [9]. The number of bacterial genes is estimated to be more than three million which far exceeds the human genome of 23,000 [10].
There remains a significant unmet need for people with IBS and individuals often struggle to receive the appropriate level of support required [11], Therapeutic interventions for IBS are described by the National Institute for Health and Care Excellence, and this guidance includes probiotics [12]. To date, only a limited number of probiotics have undergone testing in a research setting for IBS symptom management with varying results. Probiotics differ in their form (e.g. liquids, powders, capsules), composition (e.g. strains, dose, nutritional components) and not least the delivery system, which is crucial to ensure the live bacteria remain viable throughout the gastrointestinal tract and are able to navigate the gastric, pancreatic and biliary secretions unscathed before colonising the large intestine [13]. The use of probiotics in health management has instigated curiosity amongst the general public and healthcare professionals (HCPs) and was predicted to be a $46bn global market in 2020 [14]. This appears to be, in part, due to their reputation as an easily implementable strategy and established safety profile in the vast majority of individuals, with potential side effects considered mild, transient and localised to the gastrointestinal tract [15]. Furthermore, patients with IBS commonly report self-managing with non-medication therapies (e.g. nutraceuticals or ‘functional foods’).
Symprove removed for blind peer review is a water-based probiotic supplement containing liquid barley extract (substrate) and four strains of naturally occurring live bacteria: Lactobacillus rhamnosus NCIMB 30174, Lactobacillus plantarum NCIMB 30173, Lactobacillus acidophilus NCIMB 30175 and Enterococcus faecium NCIMB 30176, containing ten billion live bacteria per 70 ml dose. Previous research using the in vitro Simulator of the Human Intestine Microbial Ecosystem has demonstrated that Symprove remains viable throughout the gastrointestinal tract and colonises the luminal and mucosal layers of the large intestine [16]. A randomised, double blinded, placebo-controlled trial in patients with moderate to severe IBS showed a statistically significant beneficial effect on overall symptom severity (P=0.01) after 12 weeks of Symprove use, particularly abdominal pain (P=0.03) and bowel habit satisfaction (P=0.01) compared with controls [17].
Given the complementary contribution of real-world evidence data in supporting randomised controlled trials, which captures data in a uncontrolled, non-interventional, observational manner [18], the aim of this real-world evidence study was to evaluate the effect of Symprove on participant-reported change in IBS or IBS-like symptoms, and quality of life (QoL) after consumption for a minimum of 4 weeks.
Methods
Recruitment and eligibility
Participants were invited to take part through email and social media advertising over an 8-week period. Participation was incentivised through advertisement of a low value prize draw. Participants were only allowed to complete the online questionnaire once. After initial expression of interest, participants were informed of the study objectives. Once informed consent had been obtained and participants had agreed that their anonymised data could be used for research purposes, they were provided with a link to the online questionnaire. Participants that entered the study had a cookie installed on their browser that prevented them from entering the questionnaire more than once. Consistent with previously published real-world evidence studies, exclusion criteria included those aged under 18 years old, non-users of Symprove and non-UK residents.
The responses were captured using bespoke, digital interface software, developed and optimised for frictionless data capture and for use across all electronic devices (e.g. mobile phones, tablets, desktop computers). An initial test questionnaire was piloted prior to the main study in order to capture and resolve potential difficulties in interpretation or ambiguity with the questions presented. This data was not included in the final analysis.
Questionnaire development
The online questionnaire was developed and reviewed by an expert panel as described in previously published real-world evidence studies [19,20]. Questions used Likert-style responses where appropriate [21] and aimed to take approximately 10 minutes to complete. For example, ‘on a scale of 0 to 10 (where 0 is no pain and 10 is very severe), how severe was your abdominal (tummy) pain before/after you started taking Symprove?’ whereby 0-3 was classified as low, 4-6 was classified as medium and 7-10 was classified as high.
The online questionnaire was specifically designed to evaluate the effect of Symprove for IBS or IBS-like symptoms. Two cohorts were recruited via two pathways; medically-diagnosed IBS and self-diagnosed IBS. The descriptive terms ‘IBS’ or ‘IBSlike symptoms’ were used to reflect real-world nomenclature, as opposed to adherence of strict clinical terminology. Participants were asked whether they had experienced ‘complete resolution’ ‘some positive difference’, ‘no difference’ or ‘worsening’ of their IBS or IBS-like symptoms after trialling Symprove.
Participants completed questions that were applicable to them only. Participants were directed to particular questions depending on their previous answer. The online questionnaire requested the following information from participants:
1. Demographics and characteristics
2. Present or past use of Symprove and primary reason for using Symprove
3. Medically-diagnosed IBS or self-diagnosed IBS (including ‘alternative practitioner’ diagnosis), health status, medication use (for IBS or other health conditions), frequency of visits to a Health Care Professional (HCP)
4. IBS symptomology before and after Symprove use including abdominal pain, abdominal bloating, urgency, bowel habit satisfaction, in addition to quality of life
5. Unwanted symptoms experienced after Symprove use
Statistical analysis
P values were calculated using proportions Z test, t-test and Mann-Whitney U tests were used when data was non-parametric (stated when used). Differences are stated as statistically significant where P ≤ .05. Based on an IBS prevalence of approximately 10% [2], a power calculation indicated a sample size of 1246 at 3% margin of error would be required to give a confidence level of 95%.
Results
Overall characteristics of participants
The questionnaire was completed by 2301 individuals between 6th February and 31st March 2020. Participants with IBS or IBS-like symptoms (n=1246) were 91% (n=860) white, 69% (n=651) educated to degree level or higher, 78% (n=735) were women, 61% (n=580) were aged between 30 and 60 years and 97% (n=917) were non-smokers. 50% (n= 476) regularly purchased food from health food stores and bought organic, where possible. The demographics and characteristics questions were answered by 945 participants (41%). A large proportion elected not to answer these questions.
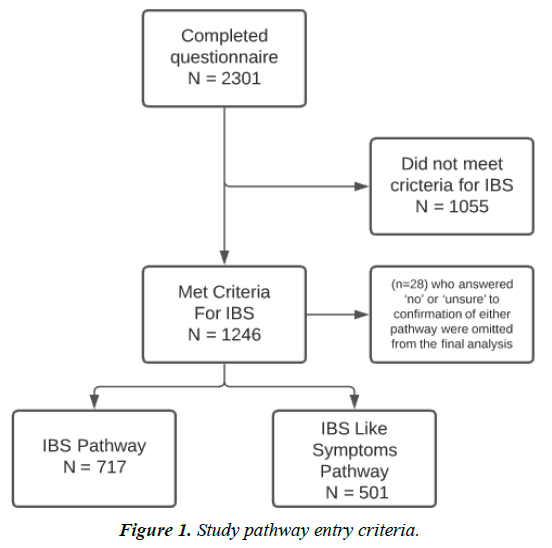
IBS or IBS-like symptoms were the primary reasons for starting Symprove in 1246 (54%). Participants could have more than one health condition. Of the 1246, 58% (n=717) entered the medically-diagnosed pathway and 40% entered the selfdiagnosed pathway (n=501). The remaining 2% (n=28) who answered ‘no’ or ‘unsure’ to confirmation of either pathway were omitted from the final analysis, Figure 1. Therefore, final reporting of results includes 1218 participants, of which 57.5% (n=717) and 42.5% (n=501) reported medically-diagnosed IBS and self-diagnosed IBS, respectively.
91% and 92% respectively, reported taking Symprove and no other probiotic supplement. Additionally, 67% (n= 480) and 68% (n=340) respectively, were taking Symprove on a continued basis. There were no significant differences in demographics between the two cohorts. Duration of gastrointestinal symptoms experienced (over 5 years) differed between the two cohorts (72%, n= 517 and 50%, n= 252 respectively; P<0.001).
IBS or IBS-like symptoms
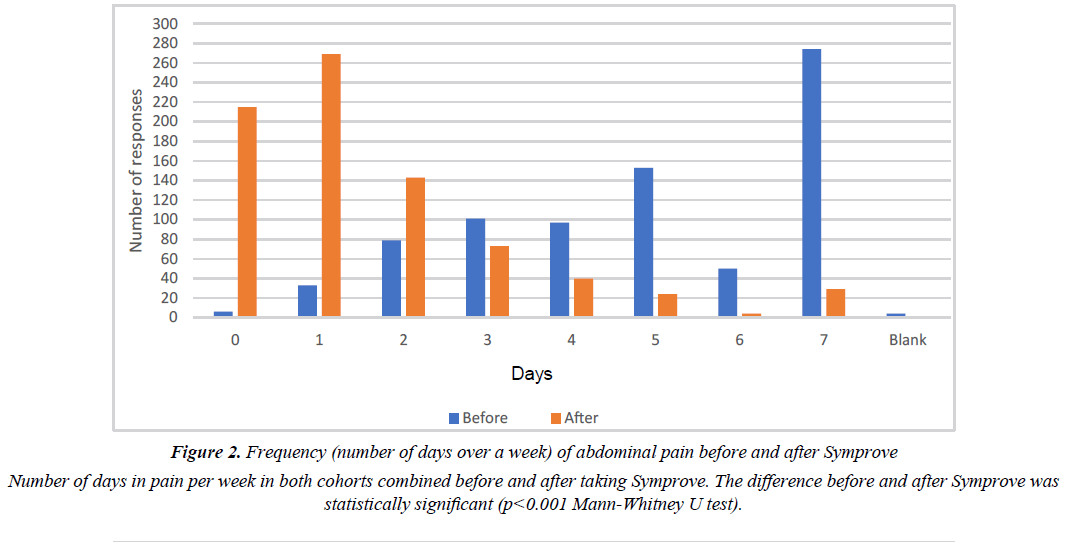
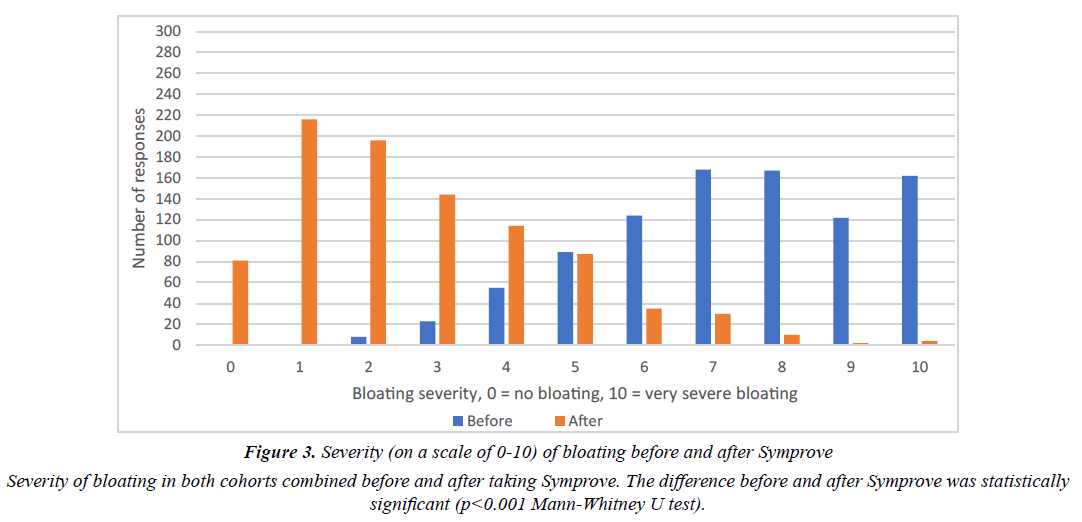
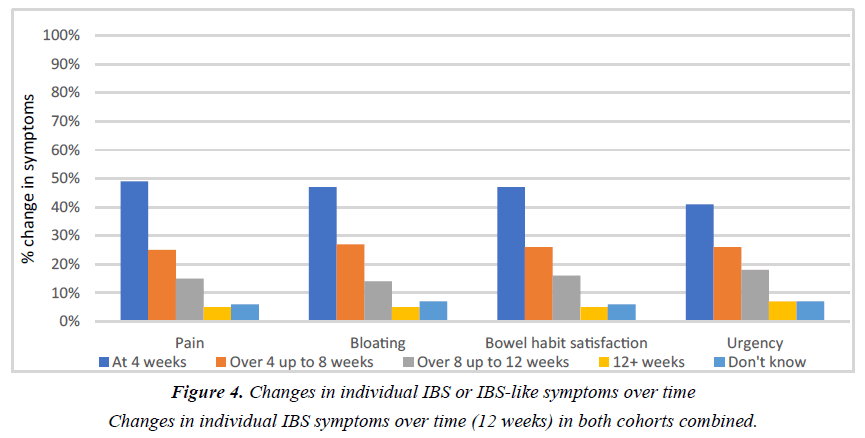
The effect of Symprove on individual IBS symptoms is shown in Tables 1 and 2. There were no significant differences between the two cohorts. Figures 2 and 3 show the frequency of abdominal pain episodes and severity of bloating in both cohorts combined. After Symprove use, there was a significant improvement in these symptoms (P<0.001, Mann-Whitney U test). Figure 4 illustrates the change in IBS symptom improvement from 4 or more weeks use of Symprove in both cohorts combined.
| After taking probiotic | Completely resolved % (total number) total responses |
Some positive difference % (total number) total responses | No difference or made symptoms worse % (total number) total responses | P value |
|---|---|---|---|---|
| Any IBS symptom/s | 12.4 (89) 717 | 76.4 (548) 717 | 9.6 (69) 717 | <0.001 |
| Pain | 19.1 (104) 544 | 75.6 (411) 544 | 2.6 (14) 544 | <0.001 |
| Bloating | 16 (90) 564 | 77.3 (436) 564 | 5.1 (29) 564 | <0.001 |
| Bowel habit satisfaction | 13.4 (75) 560 | 80.4 (450) 560 | 4.8 (27) 560 | <0.001 |
| Urgency | 15.7 (55) 350 | 76.6 (268) 350 | 5.4 (19) 350 | <0.001 |
Table 1: Effect of Symprove on IBS symptoms in participants with medically-diagnosed IBS
| After taking probiotic | Completely resolved % (total number) total responses |
Some positive difference (% total number, total responses) | No difference or made symptoms worse % (total number) total responses | P value |
|---|---|---|---|---|
| Any IBS symptom/s | 16 (80) 501 | 75.4 (378) 501 | 7.0 (35) 501 | <0.001 |
| Pain | 22.5 (64) 284 | 75 (213) 284 | 0.7 (2) 284 | <0.001 |
| Bloating | 15.6 (63) 403 | 78.1 (315) 403 | 4.7 (19) 403 | <0.001 |
| Bowel habit satisfaction | 17.4 (64) 368 | 76.9 (283) 368 | 3.8 (14) 368 | <0.001 |
| Urgency | 16.7 (29) 174 | 77.6 (135) 174 | 3.4 (6) 174 | <0.001 |
Table 2: Effect of Symprove on IBS symptoms in participants with self-diagnosed IBS.
Bloating was the most bothersome symptom, as reported by 39% (n=387), while pain (17%, n=169) and bowel habit satisfaction (20.5%, n=202) were ranked as less bothersome. Some (n=26) participants did not specify their symptom ranking.
Of the 1218, a total of 104 participants (8%) in both IBS cohorts reported ‘no difference in symptoms’ or ‘made symptoms worse’ after taking Symprove (Table 1 and 2). 27 participants reported that Symprove led to transient worsening of their symptoms including (in frequency order) worsening of bowel habit, bloating, urgency and pain, of which 70% resolved within 2 weeks.
For Table 1 the numbers in the rows do not add up to the total number of responses as a small number of participants stated that they could not recall the impact of the probiotic on a symptom. The p value refers to the differences between the total number of participants with complete resolution and some improvement in symptoms versus those where there was no effect or where symptoms worsened during Symprove consumption.
For Table 2 the numbers in the rows do not add up to the total number of responses as a small number of participants stated that they could not recall the impact of the probiotic on a symptom. The p value refers to the differences between the total number of participants with complete resolution and some improvement in symptoms versus those where there was no effect or where symptoms worsened during Symprove consumption.
Quality of life
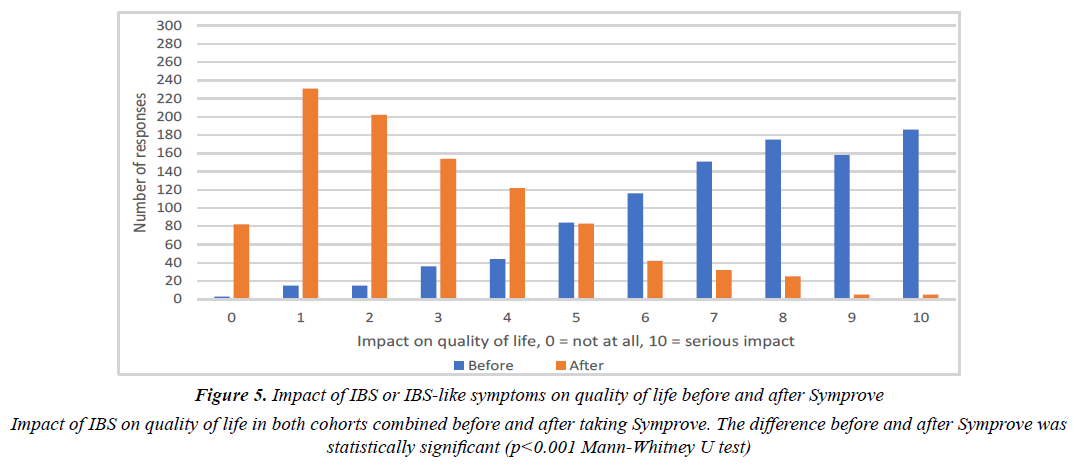
69% and 70% felt their symptoms affected their quality of life, respectively. There were no significant differences between the two cohorts. Figure 5 illustrates the impact of IBS or IBS-like symptoms on QOL. After Symprove use, there was a significant improvement in QOL for participants with very troublesome IBS or IBS-like symptoms, (self rating 7-10), 61.2% vs 6.1% (P<0.001, Mann-Whitney U test).
Medication use
26% (n=146) in the medically-diagnosed cohort had previously been prescribed or recommended medication by a HCP for their IBS. Of those, 68% (n=99) taking prescribed or recommended medication reduced the amount of medication after taking Symprove. 12% (n=39) in the self-diagnosed cohort had previously been prescribed or recommended medication by a HCP for their IBS-like symptoms. Of those, 69% (n=27) reduced the amount of medication after taking Symprove.
The most common prescription and non-prescription medications reported for management of IBS or IBS-like symptoms included Buscopan, Mebeverine, Imodium, Omeprazole and Amitriptyline.
Limitations
This real-world evidence study using questionnaires to retrospectively sample individual opinion, reaching out to product users through social media is subject to selection bias and recall bias. Using a questionnaire as the sampling tool relies on self-evaluation of symptoms, with no control data. The individuals who agreed to participate are not representative of the UK population. Our study design did not enable us to carry out an a multivariant analysis to evaluate the impact of confounding factors on our results.
Discussion
Using observational methodologies, this real-world evidence study aimed to evaluate the effect of Symprove on participantreported change in IBS or IBS-like symptoms, and QoL after consumption for a minimum of 4 weeks. The medicallydiagnosed and self-diagnosed IBS cohorts were homogeneous and did not differ significantly in regard to demographics. While there is no consensus on how long a probiotic should be trialled for, recent national guidelines published by the British Society of Gastroenterology (2021) recommend up to 12 weeks [22]. For the purposes of this real-world evidence study, IBS and IBS-like symptomology were assessed after consumption of Symprove at a minimum of 4 weeks to reflect current NICE guidance [12].
Of the 1218, a large number of participants (61% average for both cohorts combined) had experienced their IBS symptoms for over 5 years. The descriptive terms ‘IBS’ or ‘IBS-like symptoms’ were used in order to reflect real-world nomenclature. Both cohorts reported similar symptom profiles, suggesting that this descriptive term was used appropriately.
Results of the current study demonstrate that IBS or IBSlike symptoms including abdominal pain, bloating, urgency and bowel habit satisfaction improved significantly across both cohorts, in addition to QOL which appears most likely attributable to the viable bacteria in Symprove exerting physiological effects in the large intestine, as evidenced by recent in vitro research [16]. The significant improvements in IBS or IBS-like symptoms observed in the current study are consistent with a previous RCT on IBS and Symprove [17], however, the progression of symptom improvement observed differed. Indeed, there appeared to be more rapid improvements in IBS or IBS-like symptoms in the current study compared with the RCT. This may reflect differences in the severity of symptoms between the two cohorts recruited considering that in the RCT, of the 186 patients recruited, 94% had an IBS symptom severity score (IBS-SSS) of ≥175 confirming at least moderate symptom severity. Indeed, this study recruited those in the community, not in secondary care where presenting IBS or IBS-like symptoms are likely to be more severe.
Although the current Rome IV IBS criteria [1] has abdominal pain as the key diagnostic feature, in addition to a change in bowel habit, the participants in this study identified bloating as the most bothersome symptom in both cohorts. In part, the Rome IV criteria were formulated to ensure that randomised clinical trials in IBS included a relatively homogenous group of patients. It seems highly plausible that the selection bias that is inherent in many of these research design protocols differ from the real-world experiences of a health condition, including IBS, by patients and HCPs.
Symprove was associated with a reduction in the use of prescribed or recommended medication, in addition to reduced frequency of visits to HCPs. Promoting self-management through evidence-based therapies such as probiotics is likely to benefit patients (e.g. increased self-confidence, empowerment) and the wider healthcare economy. Equally, it is important to acknowledge that the overall size of the placebo effect of interventions in functional gut disorders, particularly IBS (e.g. probiotics, low FODMAP diet) is in the range of 40% [23]. A small number of participants (n= 27) also reported transient worsening of their symptoms which may be attributable, in part, due a nocebo/anticipatory response and/or potential alterations within the gut microbiome environment. This is reflective of the randomised, double blinded, placebo-controlled trial (17) whereby n= 35 and n =19 in the probiotic and placebo groups, respectively reported adverse events. A change in bowel habit and bloating were the most commonly reported.
Real-world evidence studies using observational methodologies relies on retrospective reporting of an outcome, with no associated control data [18]. However, given that over 90% of participants in the current study were only taking Symprove and no other probiotic, whilst nearly 70% were taking Symprove at the time of completing the online questionnaire, rather than relying on memory recall, it may have mitigated this impact to an extent. Recall bias and confounding factors are recognised limitations known to influence outcomes of observational studies. In particular, recruitment for the current study occurred at the beginning of COVID restrictions in March 2020. Anticipatory uncertainty, rapid adjustment to home and work life for participants, coupled with feelings of worry/anxiety were potential and unavoidable confounders.
While RCTs maintain rigour from a research design and methodological perspective as well as forming the foundation for translation from research to clinical practice, findings are often limited in their interpretability due to highly selective patient populations, thus lack of sample population representativeness and applicability in real-world populations [18]. It has been suggested real-world evidence studies are more likely to capture behaviours in a real-world setting and thus considered mutually complementary to RCTs [24]. While this study aimed to capture a sample population that was representative of the IBS population, participants were predominantly white, affluent, educated women, with a propensity to purchase organic foods, and therefore generalisability to the wider UK population (e.g. male, ethnic groups, etc.) is limited. Furthermore, it is not possible to eliminate selection bias (i.e. those who experienced a positive effect opted to complete the questionnaire). Equally, in accordance with previous systematic reviews and metaanalyses [25,26], the majority of RCTs that have investigated probiotic use for IBS management are small in sample size, typically under 100 patients. The current study demonstrates how significantly larger sample sizes can be recruited through email and social media advertising.
The descriptive terms ‘IBS’ or ‘IBS-like symptoms’ were used in order to reflect real-world nomenclature, as were the fluid nature of some of the questions asked (e.g. did taking Symprove make a difference to your symptoms? Options included ‘completely resolved’, ‘some positive difference’, ‘no difference/made symptoms worse’. Both cohorts reported similar symptom profiles, suggesting that the descriptive term ‘IBS’ or ‘IBS-like symptoms’ were used appropriately. Given the overlap and manifestation of IBS or IBS-like symptoms in other gastrointestinal disorders, it’s plausible that some symptoms reported in the self-diagnosed pathway by participants may have been reflective of a separate condition (e.g. lactose intolerance, wheat sensitivity).
Current IBS management encompasses medical, dietary, nutraceutical and psychological approaches all with varying levels of efficacy [26-28], which reflect the complex and multifactorial nature of IBS pathophysiology. While medical management is often considered a first-line strategy, many IBS medications have side-effects and target individual symptoms when the reality for patients with IBS is the presence of multiple concurrent symptoms.
In clinical practice, a prescriptive approach to recommending probiotics is warranted since appropriate probiotic selection is crucial in order to maximise any potential symptomatic improvements for patients with IBS or IBS like symptoms [29,30]. This study provides evidence for the beneficial effect of Symprove in individuals with IBS or IBS-like symptoms and may be considered a potentially useful adjunct in the integrated management of patients with IBS in primary and secondary care.
Acknowledgement
Thanks to Tricia Beaumont, Nick Hayhoe, Dr. Sathish Sankarpandi, Barry Smith and Peter Brady for their valuable contributions in designing and interpreting this study.
Funding Information
This study was supported by Symprove Ltd.
Conflict of Interests
Simon Rudland is the medical adviser to Orbital Global Research, who conducted this research. Professor Bjarnason has been the principal investigator for three clinical studies that were supported by Symprove Ltd via unrestricted grants to King’s College Hospital.
References
- Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393-1407.
- Wilson S, Roberst L, Rolfe A, et al. Prevalence of irritable bowel syndrome: a community survey. Br J Gen l Pract. 2004;54:495-502.
- Spiegel B. The burden of IBS: Looking at metrics. Currt Gastroenterol Rep. 2009;11(4):265-269.
- Drossman DA. The Rome IV Committees, editor. History of functional gastrointestinal symptoms and disorders and chronicle of the Rome Foundation. In: Drossman DA, Chang LC, Kellow WJ, Tack J, Whitehead WE, editors. Rome IV functional gastrointestinal disorders: disorders of gut-brain interaction. I. Raleigh, NC: The Rome Foundation. 2016;549-576.
- Smith G, Steinke D, Kinnear M, et al. A comparison of irritable bowel syndrome patients managed in primary and secondary care: The Episode IBS study. Br J Gen Pract. 2004;54(504):503-507.
- Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—A Systematic Review. Gastroenterology. 2019;157(1);97-108.
- Schmulson M, Drossman D. What Is New in Rome IV. J Neurogastroenterol Motil. 2017;23(2):151-163.
- Valdes AM, Walter J, Segal E, et al. Role of the Gut Microbiota in Nutrition and Health. BMJ. 2018;361:k2179.
- Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14(8):e1002533.
- Vyas U, Ranganathan N. Probiotics, prebiotics, and synbiotics: gut and beyond. Gastroenterol Res Pract. 2012;2012:872716.
- Corsetti M, Whorwell P. New therapeutic options for IBS: the role of the first in class mixed µ- opioid receptor agonist and δ-opioid receptor antagonist (mudelta) eluxadoline. Expert Rev Gastroenterol Hepatol. 2017;11(4):285-292.
- The National Institute for Health and Care Excellence. (2019). Irritable bowel syndrome in adults: diagnosis and management.
- Fredua-Agyeman M, Gaisford S. Comparative survival of commercial probiotic formulations: tests in biorelevant gastric fluids and real-time measurements using microcalorimetry. Benef Microbes. 2015; 6(1):141-151.
- O'Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
- Hempel S, Newberry S, Ruelaz A, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess. 2011;200:1-645.
- Moens F, Van den Abbeele P, Basit A, et al. A four-strain probiotic exerts positive immunomodulatory effects by enhancing colonic butyrate production in vitro. Intl J Pharmaceut. 2019;555:1-10.
- Sisson G, Ayis S, Sherwood RA, et al. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome--a 12 week double-blind study. Aliment Pharmacol Ther. 2014;40(1):51-60.
- Sherman R, Anderson S, Dal Pan G, et al. Real-World Evidence-What is it and what can it tell us? . N Engl J Med. 2016;375(23):2293-2297.
- Goldman M, & Beaumont T. A real world evaluation of a treatment for infant colic based on the experience and perceptions of 4004 parents. Br J Nurs. 2017;26(5):S3-S10.
- Goldman M, & Lodhi I. A real-world evidence study evaluating a treatment for nappy rash. Br J Nurs. 2016;25(8):432-439.
- Joshi A, Kale S, Chandel S. Likert Scale: Explored and Explained. JAST, 2015;7(4):396-403.
- Vasant DH, Paine PA, Black CJ, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;324-598.
- Enck P, Klosterhalfen S. Placebo Responses and Placebo Effects in Functional Gastrointestinal Disorders. Front Psychiatry. 2020;11:797.
- Kim H, Lee S, & Kim J. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J Korean Med Sci. 2018;33(34):e213.
- Ford AC, Harris L, Lacy B, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther, 2018;48(10):1044-1060.
- Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561.
- Black CJ, Thakue ER, Houghton L, et al. Efficacy of psychological therapies for irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2020;69(8):1441-1451.
- Cook R, Davidson P, Martin R. Telephone or internet delivered talking therapy can alleviate irritable bowel symptoms. BMJ. 2019;367:l4962.
- Hoveyda N, Heneghan C, Mahtani K, et al. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15.
- Li B, Liang L, Deng H, et al. Efficacy and Safety of Probiotics in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Front Pharmacol. 2020;11:332.