Research Article - Biomedical Research (2017) Volume 28, Issue 17
A method of rapid rabbit atherosclerosis model establishment
Yinfei Qiao1, Kejian Zhu2, Zhengping Bo2, Zhengke Yu1, Yuming Han1, Qi Zhao1, Wenli Yang1, Xiangyi Xia1, Ting Zhang1 and Changjiang Xiao1*
1Department of Cardiology, Affiliated Hospital of Hunan Academy of Chinese Medicine, Changsha City, Hunan Province, 410006, PR China
2Department of Outpatient, Affiliated Hospital of Hunan Academy of Chinese Medicine, Changsha City, Hunan Province, 410006, PR China
- *Corresponding Author:
- Changjiang Xiao
Department of Cardiology
Affiliated Hospital of Hunan Academy of Chinese Medicine, PR China
Accepted date: August 17, 2017
Abstract
To establish a method to produce the atherosclerosis rabbit model rapidly. A total of 18 healthy male Japanese white rabbits were randomly divided into the control group (n=8) and the experiment group (n=10). Rabbits in control group were fed with regular chow diet, while rabbits in the experiment group were fed with high fat diet and injected with bovine albumin. All animals received injure on left femoral arteries by vessel sheath. After seven weeks of injury and diet, morphological characters of pathological changes were investigated, serum lipoprotein and cholesterol levels were also profiled. Serum total cholesterol (TC), triglyceride (TG), and low density lipoprotein (LDL) levels were significantly higher in experiment group than control group (P<0.05). All rabbits in experiment group developed atherosclerotic plaques in left femoral arteries where was injured by vessel sheath indicating the successful establishment of the rabbit atherosclerosis model. The combination of high fat diet, intravenous injection of bovine albumin and arterial injury resulted in a successful establishment of atherosclerosis model in rabbit within a relatively short period of time.
Keywords
Atherosclerosis, Rabbit model, Bovine albumin, Balloon injury
Introduction
Atherosclerosis (AS) is a chronic inflammatory disease in which various types of inflammatory cells are involved [1]. Atherosclerosis initiates as a result of damage to endothelial cells (ECs) which subsequently promotes adherence of monocytes to ECs [2]. Monocytes migrate into endothelium, where they differentiate into macrophages, which engulf oxidize low-density lipoproteins (LDLs) and constitute important steps in atherosclerosis development [3]. As a result, residual macrophages and smooth muscle cells turn into foam cells and further facilitate atherosclerotic plaque formation. Histologically, atherosclerotic plaques are composed of fibrous caps, which are prone to breaking and thus causing coronary artery lumen stenosis and thrombosis once unstable plaque ruptures. The occurrence of AS is associated with numerous risk factors, such as dyslipidemia and local hemodynamic changes like shear stress [4-6].
A variety of small and large animal models have been used to study the pathogenesis of AS [7]. However, none of the animal atherosclerosis models is ideal because each model has its own advantages and disadvantages. Due to the ease of genetic manipulation, murine models are currently most extensively used [8]. However, murine atherosclerosis is not identical to humans in various aspects. Besides large animal models include pigs, rabbits, and primates are not well established and genetic manipulation has still been a challenge. Nevertheless, large animal models have particular advantages for certain types of studies and potential similarity to human atherosclerotic lesion morphology [9].
Rabbit is the species which is the most sensitive model to dietary cholesterol overload and is extensively used as AS model. The Watanabe hereditary hypercholesterolemic (WHHL) rabbit has a defect in the low density lipoprotein receptor (LDLR) and are prone to develop the advanced lesions in a short period of time [10]. The most important point is that the pathological process of rabbits is very similar to humans. Therefore, rabbits have been extensively used as a suitable AS model, but need relatively longer period of diet feeding to induce atherosclerosis development. Therefore, the present study was designed to establish a rapid rabbit atherosclerosis model by combining multiple approaches, including high fat diet, injection of bovine albumin and balloon injury of artery.
Methods
Reagents
Cholesterol added in diet was purchased from Lixin Biological Technology Co., LTD (Changsha, Hunan, China) and Bovine serum albumin was purchased from Haoyang Biological Technology co., LTD (Tianjin, China). Vascular sheath (4F, 5F, 6F), radial artery puncture needle, and guide wire were all provided by Beijing Maiteli Technology Development co., LTD, (Beijing, China). Serum total cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL) tests was performed by Hunan Academy of Traditional Chinese Medicine affiliated hospital clinical laboratory using commercially available kits. Pathology staining and image analysis was performed by Hunan Academy of Traditional Chinese Medicine affiliated hospital pathology department in a blind fashion.
Experimental animals
A total of 18 male Japanese big ear rabbits weighting 2.0 ± 0.2 kg were purchased from Hunan University of Chinese Medicine Experimental Animal Center. Eighteen rabbits were randomly assigned into experimental group (n=10) and control group (n=8). All rabbits were separately caged and had free access to water. The rabbits in control group were fed with 150 g regular diet daily for a total of seven weeks. The rabbits in experimental group were fed with high-fat diet (2% cholesterol, 10% lard, 10% egg yolk powder, 0.2% propylthiouracil, and 67.8% regular diet) with50 g high-fat diet in the morning and 100 g regular diet in the afternoon daily for a total of seven weeks.
Establishment of atherosclerosis model
The rabbits in experimental group were injected with 250 mg/kg bovine albumin via ear veins at the 1st and 7th days of high fat diet. Besides, rabbits in experimental group received femoral artery injury under general anesthesia on day 1 of high fat diet. In brief, rabbits were anesthetized with intramuscular injection of 3% pentobarbital sodium (30 mg/kg). Left inguinal area was shaved and skin was disinfected. Femoral artery was separated and exposed. 21 G radial artery puncture needle was inserted into the middle and lower sections of femoral artery. With the aid of guide wire, radial artery sheath was inserted towards 5 centimeter (cm) depth (4F, 5F, and 6F sheathes were chosen to be used on rabbits weighting1.8 kg, 2.0 kg, and 2.2 kg respectively). Then all rabbits were injected with 200 U/Kg heparin via ear margin veins. After the guide wires of the sheath were withdrawn, the arterial sections with sheath were pressed, the sheath with dynamometer was pull out for 4 cm. The force of press was 10 Newton as demonstrated on dynamometer. This was repeated for 3 times to cause vascular endothelial damage. The sheath was then completely pulled out to finish the injury. Yunnan Baiyao 1 g was spilled on the puncture area which was subsequently compressed for 5 min to stop bleeding. After bleeding was stopped, gentamicin 80000 U was spilled on the incision area to prevent infection. The incision was closed by conventional layered sutures. All rabbits were given 400,000 units of penicillin daily for consecutive 3 days to prevent infection. All rabbit were fast 12 h before the procedure.
Collection and measurement of specimen
Blood sample was collected after 12 h fasting before the injury, on the 3rd and the 7th weeks after the injury. At 7th week time point, all rabbits were sacrificed by air embolism. The left femoral arteries were harvested, flushed with normal saline and kept in 10% formalin solution for pathology investigation. All the samples were embedded in paraffin, cross-sectioned, and stained with H&E. Light microscope was used to observe pathological changes.
Statistical analysis
Statistical analysis was performed using SPSS 16.0. The data were expressed as mean ± standard deviation (SD). Differences were analyzed by one-way analysis of variance (ANOVA) followed by the Newman-Keuls test. P<0.05 were considered as statistically significant.
Results
General situation
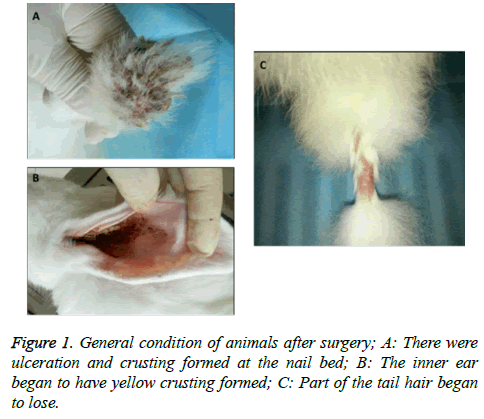
All rabbits in control group were in good condition with white and shining furs, bright eyes and responsive to feeding and active in scratching cages. No mortality was observed in control group. All rabbits in experimental group began to show poor spirit and became less active at the 3rd week of experiment/injury. Although having normal appetite, they were less responsive to feeding on the 5th week and tended to be lying rather than being active. Their appetite further decreased and fur became dark. There were ulceration and crusting formed at nail beds of four extremities (Figure 1A). The inner ear began to appear yellow crusting (Figure 1B) and experienced hair lost (Figure 1C). On the 7th week, they began to have cloudy eyes and fat deposition. One rabbit suddenly died in the middle of the 7th week without significant abnormalities found by autopsy.
Measurement of serum lipids
There was no significant difference of TG, TC and LDL levels before experiment, 3rd week and the 7th week after injury (all P>0.05) (Table 1) in control group. In contrast, serum lipid levels including TG, TC, and LDL of experiment group were significantly increased at the 3rd and 7th weeks compared with pre-experiment (TG, 1.49 ± 1.02, 2.44 ± 0.57 vs. 0.64 ± 0.24; TC, 33.98 ± 2.13, 45.79 ± 1.69 vs. 0.92 ± 0.27; LDL, 28.83 ± 1.97, 33.04 ± 2.17 vs. 0.51 ± 0.23) (P<0.01) (Table 1), suggesting a successful induction of hyperlipidemia in experiment group.
| Lipids (mmol/L) | Before experiment | 3rd week | 7th week | |||
|---|---|---|---|---|---|---|
| Control | Experiment | Control | Experiment | Control | Experiment | |
| TG | 0.59 ± 0.28 | 0.64 ± 0.24 | 0.61 ± 0.13 | 1.49 ± 1.02*^ | 0.64 ± 0.19 | 2.44 ± 0.57**^ |
| TC | 0.89 ± 0.20 | 0.92 ± 0.27 | 0.92 ± 0.41 | 33.98 ± 2.13*^ | 0.91 ± 0.33 | 45.79 ± 1.69**^ |
| LDL | 0.56 ± 0.13 | 0.51 ± 0.23 | 0.59 ± 0.39 | 28.83 ± 1.97*^ | 0.54 ± 0.41 | 33.04 ± 2.17**^ |
Table 1. Measurement of serum lipids at the different time points of two groups.
Observation of pathological morphology
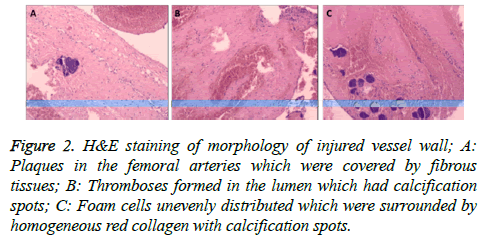
Endothelium was complete and intact in the femoral arteries in the control group, which was single-layered and close to the inner elastic plate. The smooth muscle cells in the middle layer were in oval shape. In contrast, plaques were observed in the injured femoral arteries in experiment group which were covered by fibrous tissues. There was also thromboses formation in the lumen where calcification spots were observed. Thickness of arterial wall was not identical to that of the control group although one side of the vessel wall became thinner with smooth muscle rupture. The other side of it had foam cells unevenly distributed surrounded by homogeneous red collagen and calcification spots. There was arterial bleeding and hematoma formation between the middle and the outer layer (Figures 2A-2C).
Figure 2: H&E staining of morphology of injured vessel wall; A: Plaques in the femoral arteries which were covered by fibrous tissues; B: Thromboses formed in the lumen which had calcification spots; C: Foam cells unevenly distributed which were surrounded by homogeneous red collagen with calcification spots.
Discussion
The pathological process of AS mainly involves large- and middle-sized arteries [11]. Although the exact pathogenesis or mechanisms of atherosclerosis development are still unclear, the damage response theory and inflammatory theory are the two most widely accepted ones [12]. Endothelial cells are damaged which results in vascular inflammation and subsequent formation of foam cells and fibrous tissue proliferation. Pathology studies show the formation of fatty streak, fibrous tissues, and ultimate plaques formation in the different stages of atherosclerosis development. Abnormal lipid metabolism, especially serum lipids such as TG, TC, and LDL is one of the most important factors in the progress of AS [13]. It is not only the initial step in AS but also plays an important role in leading vascular endothelial damage [14]. The establishment of AS model using high-fat diet plus balloon injury on abdominal or iliac arteries has been extensively used. Some studies have reported that high-fat diet plus inflammatory stimulus is able to produce AS model [15]. However, all these methods are relatively time-consuming. To provide a better animal model which could not only mimic the atherosclerosis formation in human, but also have a relatively shorter period in model establishment, we have combined several approached that have been used in large atherosclerosis animal model establishment.
In the current study, the expensive balloon plus pump method was abandoned due to its high cost. Instead, relatively costeffective vascular sheath plus dynamometer method was adopted. During the femoral artery homeostasis, Yunnan Baiyao powder plus 5-minute compression method was used instead of using gelatin sponge dressing method to stop bleeding. By adopting the above methodologies, the cost has been reduced to 1/20 of the original one, operation time was reduced, infection rate and mortality were also decreased.
High-fat diet can directly cause excessive intake of lipids resulting in hyperlipidemia [16]. Propylthiouracil can cause thyroid dysfunction and slow disturbed lipid metabolism in rabbits [17]. Intravenous injection of bovine serum albumin can cause systemic immune inflammatory response [18]. Vascular sheath injury can directly cause endothelial injury in rabbits [19]. We proposed that the combination of the above four methods can result in a three-dimensional interaction of complex biological effects, so that it could accelerate the atherosclerosis development. Therefore, we have adopted a new model of local artery injury using vascular sheath on the basis of systemic reactions caused by high-fat diet, propylthiouracil and bovine serum albumin. We found that rabbits in control group were in generally good condition, while rabbits in experiment group gradually appear a variety of symptoms including blood lipid level elevation, AS plaque formation and partial vessel wall calcification, indicating the successful establishment of AS model within seven weeks. The traditional atherosclerosis rabbit model including either highfat diet, systemic inflammation, or balloon injury usually require eight weeks for animal model establishment. The present method has shortened the time to some extent.
There are also several key steps which are required to be paid attention throughout the whole process. First of all, according to our experience, to successfully establish this rapid atherosclerosis model, the age and body weight of animal are of great importance. Therefore, to achieve a reproducible result, researchers should use animal with similar age and body weight as used in the current study. Second, the dosage of bovine serum albumin injection should be sufficiently calculated. To accomplish this, the leaking of injected solution into local tissue around the ear vein should be avoided in order to prevent local tissue necrosis and the loss of albumin. Third, anesthetic dosage should be appropriate to prevent death of rabbits due to overdose. Intramuscular injection method is the best choice to prevent overdose. Fourth, all surgical instruments should be heparinized to prevent small thrombosis formation. Fifth, during vascular sheath injury, operator should pay special attention to the dynamometer reading to prevent excessive force resulting in severe damage of the artery wall, the possible blood vessels rupture, or even death of animals. Excessive force can also cause elastic plate rupture leading to more scars formation and resulting in failure of model establishment. Sixth, vascular sheath should be pull out quickly in the end of the injury procedure and Yunnan Baiyao powder should be spilled in the vascular puncture areas to stop bleeding as quick as possible.
In conclusion, compared with conventional method, the present method has combined several approached used in rabbit atherosclerosis model establishment and leads to a relatively rapid development of atherosclerosis plaque in rabbit. This animal model has several advantages including time-saving, cost-effective, and high success rate which should be widely applied in the AS studies.
Acknowledgment
This work was supported by grants from The 2014 College Scientific Research Fund Project in Hunan Academy of Chinese Medicine (Funding NO. 201403) and The “2013 National Famous TCM Expert Inherit Studio” Funding Project of State Administration of Traditional Chinese Medicine of the People’s Republic of China.
References
- Chapman MJ, Blankenberg S, Landmesser U. The year in cardiology 2015: prevention. Eur Heart J 2016.
- Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol 2015; 6: 365.
- Conti P, Shaik-Dasthagirisaeb Y. Atherosclerosis: a chronic inflammatory disease mediated by mast cells. Cent Eur J Immunol 2015; 40: 380-386.
- Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol 2015.
- Pende A, Artom N, Bertolotto M. Role of neutrophils in atherogenesis: An update. Eur J Clin Invest 2015.
- Kurdi A, De Meyer GR, Martinet W. Potential therapeutic effects of mTOR inhibition in atherosclerosis. Br J Clin Pharmacol 2015.
- Zhang J, Wang D, He S. Roles of antibody against oxygenized low density lipoprotein inatherosclerosis: recent advances. Int J Clin Exp Med 2015; 8: 11922-11929.
- Barter PJ, Rye KA. Targeting high-density Lipoproteins to reduce cardiovascular risk: What is the evidence? Clin Ther 2015; 37: 2716-2731.
- Rojas J, Salazar J, Martínez MS. Macrophage heterogeneity and plasticity: impact of macrophage biomarkers on atherosclerosis. Scientifica (Cairo) 2015; 2015: 851252.
- Gu HM, Zhang DW. Hypercholesterolemia, low density lipoprotein receptor and proprotein convertase subtilisin/kexin-type 9. J Biomed Res 2015; 29: 356-361.
- Boesen ME, Singh D, Menon BK. A systematic literature review of the effect of carotidatherosclerosis on local vessel stiffness and elasticity. Atherosclerosis 2015; 243: 211-222.
- Fishbein MC, Fishbein GA. Arteriosclerosis: facts and fancy. Cardiovasc Pathol 2015; 24: 335-342.
- Kataoka Y, Puri R, Nicholls SJ. Inflammation, plaque progression and vulnerability: evidence from intravascular ultrasound imaging. Cardiovasc Diagn Ther 2015; 5: 280-289.
- Husain K, Hernandez W, Ansari RA. Inflammation, oxidative stress and renin angiotensin system inatherosclerosis. World J Biol Chem 2015; 6: 209-217.
- Frolow M, Drozdz A, Kowalewska A. Comprehensive assessment of vascular health in patients; towards endothelium-guided therapy. Pharmacol Rep 2015; 67: 786-792.
- Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res 2015; 116: 884-894.
- Wen YD, Zhu YZ. The Pharmacological Effects of S-Propargyl-Cysteine, a Novel Endogenous H2S-Producing Compound. Handb Exp Pharmacol 2015; 230: 325-336.
- He R, Qu AJ, Mao JM. Synergistic proliferation induced by insulin and glycated serumalbumin in rat vascular smooth muscle cells. Sheng Li Xue Bao 2007; 59: 1-7.
- Janoudi A, Shamoun FE, Kalavakunta JK. Cholesterol crystal induced arterial inflammation and destabilization of atherosclerotic plaque. Eur Heart J 2015.