Research Article - Biomedical Research (2017) Volume 28, Issue 5
A meta-analysis of the effectiveness of hypertonic saline in treating Chinese mainland populations with high intracranial pressure
Lei Feng1#, Jun Liu1#, Chunhai Su1, Yunzhen Liu1, Chuanfeng Lv2*1Division of Vascular Diseases, Department of Neurosurgery, Jining No.1 People's Hospital, Jining 272011, PR China
2Department of Clinical Pharmacy, Jining No.1 People's Hospital, Jining 272011, PR China
#These authors contributed equally as co-first authors
- *Corresponding Author:
- Chuanfeng Lv
Department of Clinical Pharmacy
Jining No.1 People's Hospital Jining, PR China
Accepted on October 10, 2016
Abstract
This study aimed to evaluate the effectiveness of hypertonic saline (HS) in treating Chinese mainland populations with high intracranial pressure (HIP). The PubMed, Medline, Cochrane Library, Elsevier, China Biology Medicine disc, China National Knowledge Infrastructure, and Wanfang databases were searched for all randomized controlled trials of HS used to treat Chinese mainland populations with HIP for inclusion in the meta-analysis. The mean difference (MD) and 95% confidence intervals (95% CI) were then calculated. A total of eight randomized controlled trials that studied a total of 567 cases were included. The results showed that compared with the control groups, the MD values of intracranial pressure in the HS groups 1 and 3 hours after administering the medication were 0.00 (95% CI: -0.30-0.30) and -0.23 (95% CI: -0.73-0.26), respectively, and no significant difference was apparent; the MD values of mean arterial pressure and mean venous pressure 1 and 3 hours after administering the medication also showed no significant difference. HS had the same effectiveness in lowering intracranial pressure and changing the hemodynamic features as other treatment options.
Keywords
Hypertonic saline, Increased intracranial pressure, Brain edema, Meta-analysis.
Introduction
Increasing intracranial pressure is one common clinical manifestation of brain trauma, including brain surgery, cerebral hemorrhage, craniocerebral trauma, intracranial spaceoccupying lesions, and intracranial infections, and is part of the pathological process of neurological diseases [1,2]; Therefore, controlling IP is key to improving patient prognosis. Normal IP in the supine position is about 1.33 kPa (10 mmHg), and when increasing PI is caused by brain tissue swelling, intracranial space-occupying lesions, excessive secretion or malabsorption of cerebrospinal fluid, blocked circulation, or excessive intracranial cerebral perfusion [3], dehydration is the main measure to prevent the development of cerebral edema, decrease IP, and prevent herniation of the brain [4]. Mannitol has been used clinically since 1962 and is currently the firstline drug for reducing IP; however, after promotion of its clinical applications, its adverse reactions have received increasing attention. Repeated administration of mannitol can lead to acute renal failure, electrolyte imbalance, and even cerebral edema aggravation (i.e., the rebound phenomenon), thus directly activating the apoptosis of brain cells and increasing patient mortality [5].
In recent years, the role of hypertonic saline (HS) in reducing IP has been re-assessed [6-8], and animal experiments and clinical studies have all shown that HS can reduce IP through various mechanisms, including optimizing the hemodynamics of the body and brain, reducing cerebral edema, regulating vascular spasm, and changing the neurochemical and immune activities of brain tissues. HS has better IP reducing effects than mannitol, and its adverse effects are significantly less than those of mannitol. A meta-analysis by Kamel et al. [9] showed that in the UK, France, or Germany, HS could more effectively reduce IP than mannitol; the main ethnic groups included in that study were European, so HS’s effects on Chinese mainland populations, mainly patients of Mongolia and Southeast Asian ethnicity, are not clear. Accordingly, this study analyzed the randomized controlled trials that compared the effectiveness of HS and other measures in treating IP, aiming to evaluate the therapeutic effects of HS in Chinese mainland populations with high intracranial pressure (HIP).
Materials and Methods
Search strategies
For English studies, the Elsevier, PubMed, Medline, and Cochrane Library databases were searched for relevant studies, and the China National Knowledge Infrastructure (CNKI), China Biology Medicine disc, and Wanfang databases were searched for Chinese language studies; the publication date was limited from the building of the database until January 2016. The search used the keywords "hypertonic saline solution," "intracranial hypertension," and "brain edema”; the languages were limited to English and Chinese, the trial type was limited to randomized controlled trials, and animal trials were excluded. The references listed in the included studies were examined, and if necessary, hand-retrieval was performed to ensure all of the relevant studies were included in this metaanalysis.
Inclusion criteria
The studies included had to meet the following conditions: 1) a randomized controlled trial; 2) without sex restrictions, patients ≥ 18 years of age, Chinese mainland residents; 3) without any primary disease limits, but accompanied by increasing PI; 4) with sustained IP monitoring, and the IP changes 1 and 3 hours after administering the medication were the primary outcomes; 5) reported data on the outcomes of interest; 6) with clear end point events and evaluation indexes.
Exclusion criteria
Exclusion criteria were: 1) Did not meet the inclusion criteria; 2) Research about drug metabolism and kinetics, animal or cell assay, or immunology; 3) Non-prospective or non-randomized controlled clinical trial; 4) Data could not be extracted due to incomplete information; 5) Prospective randomized trial without a control group or an inappropriate control group; 6) Observational study.
Main and secondary outcomes
Main outcome indicators: 1) IP 1 hour after administering the medication; 2) IP 3 hours after administering the medication. Secondary outcome indicators: 1) Mean arterial pressure 1 hour after administering the medication; 2) Mean arterial pressure 3 hours after administering the medication; 3) Mean venous pressure 1 hour after administering the medication; 4) Mean venous pressure 3 hours after administering the medication.
Literature study quality and data extraction
Two reviewers independently screened all of the studies in accordance with the inclusion and exclusion criteria and used the Jadad rating scale to assess the quality of the studies. The literature quality was mainly evaluated from the three main aspects of the Jadad rating scale, namely the randomization and allocation methods, whether a double-blind method was used, and trial dropouts and follow-up losses. Studies with a total score of 1-3 points were defined as low quality, and those with 4-7 points as high quality.
Two reviewers independently extracted the data from the included studies, and the extracted data included the basic information of the study, such as the first author and publication year, research methods, cases in the experimental group and the control group, usage of HS, and outcome indicators. After all of the data were completely extracted, these two reviewers cross-checked the data, and any differences were solved through negotiations; if the difference could not be resolved, a third party was consulted.
Statistical analysis
RevMan5.3 statistical software (provided by the Cochrane Collaboration) was used, and the continuous variables were evaluated using the mean difference (MD) and 95% confidence interval (CI). The χ2 test was performed to analyze the heterogeneity of the results and the I2 test was used to quantitatively analyze the heterogeneity, with I2>50% suggesting the presence of heterogeneity among different studies. If no heterogeneity was found among the studies, a fixed-effects model was selected for the meta-analysis; if heterogeneity existed, a random-effects model was selected. The treatment effects were reported using the MD and 95% CI, with P<0.05 considered as a significant difference. RevMan5.3 was used to generate a funnel plot to determine the existence of publication bias.
Results
Characteristics and qualities
A total of 211 articles were retrieved, and eight were finally included after applying the inclusion and exclusion criteria [10-17] (Figure 1), involving a total of 567 patients (283 cases in control groups and 284 cases in experimental groups). The general characteristics of the included trials are shown in Table 1.
| Study | Protopathy | Patient | Control Group | Hypertonic Saline (HS) Group | Jadad Score |
|---|---|---|---|---|---|
| Yuhang et al. [10] | Hypertensive Cerebral Hemorrhage | Elder | 20%mannitol | 7.5% HS | 3 |
| Ming et al.[11] | Craniocerebral Injury | Adult | 20%mannitol | 3% HS | 3 |
| Jie et al.[12] | Cerebral Infarction | Adult | 20%mannitol | 3% HS | 3 |
| Liansheng et al.[13] | Encephaledema | Adult | 20% mannitol+furosemide | 7.5% HS | 3 |
| Jianhua et al.[14] | Traumatic brain injury | Adult | 20%mannitol | 4.2% HS | 3 |
| Zhigang et al.[15] | Traumatic brain injury | Adult | 20%mannitol | 4.2% HS | 3 |
| Fanyuan et al.[16] | Traumatic brain injury | Adult | 20%mannitol | 7.5% HS | 3 |
| Yangbo et al.[17] | craniocerebral injury | Adult | 20%mannitol | 7.5% HS | 3 |
Table 1. General characteristics and Jadad score of the clinical trials included in this Meta-analysis.
Impact of HS on IP
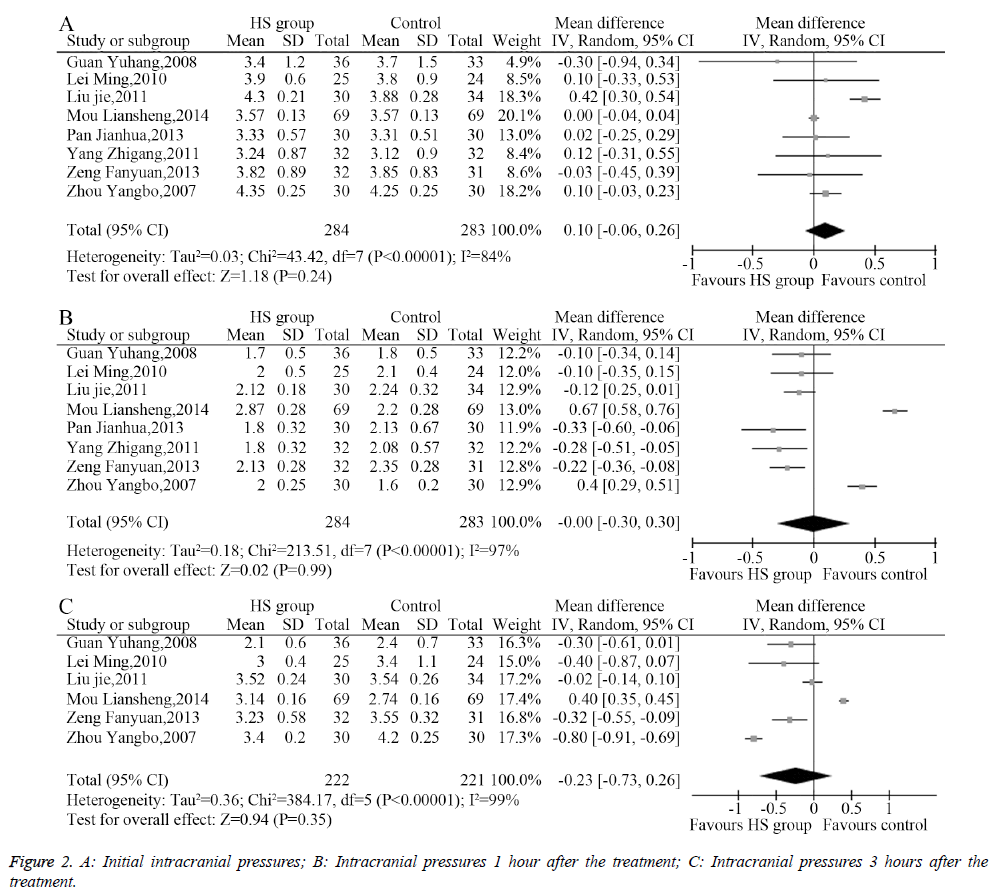
The heterogeneity analysis revealed the existence of heterogeneity among the eight included studies, so a randomeffects model was used to combine the data. The results of the meta-analysis showed no significant difference (MD=0.10, 95% CI: -0.06-0.26, P=0.24, Figure 2A) between the initial intracranial pressure in the HS group and the control group. Similarly, IP after 1 hour of treatment (MD=0.00, 95% CI: -0.30-0.30, P=0.99, Figure 2B) and 3 hours of treatment (MD=-0.23, 95% CI: -0.73-0.26, P=0.35, Figure 2C) showed no significant difference.
Effects of HS on mean arterial pressure
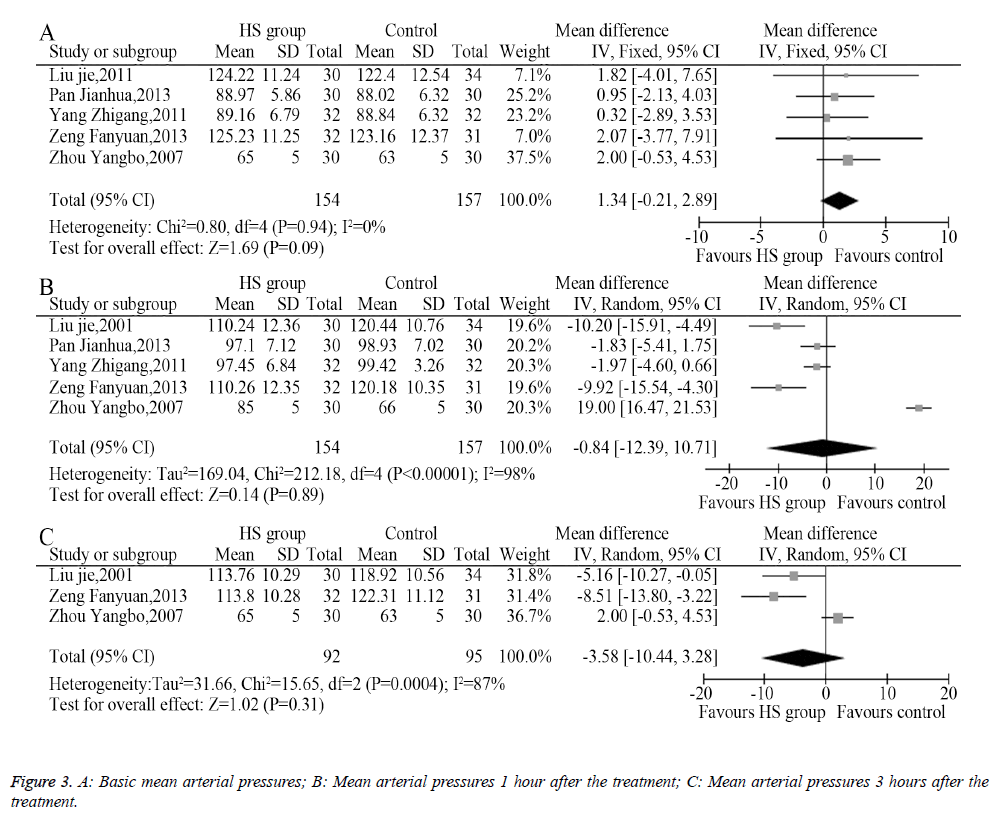
Among the eight included trials, five described the changes of mean arterial pressure an hour after administering the medication, among which three described the changes of mean arterial pressure 3 hours after administering the medication. The heterogeneity analysis provided suitable models for the combination of the included trials, and the meta-analysis showed that the pre-treatment mean arterial pressures between the HS group and the control group had no significant difference (MD=1.34, 95% CI: -0.21-2.89, P=0.09, Figure 3A). The mean arterial pressure 1 hour after treatment (MD=-0.84, 95% CI -12.39-10.71, P=0.89, Figure 3B) and 3 hours after treatment (MD=-3.58, 95% CI: -10.44-3.28, P=0.31, Figure 3C) showed no statistical difference.
Effect of HS on mean venous pressure
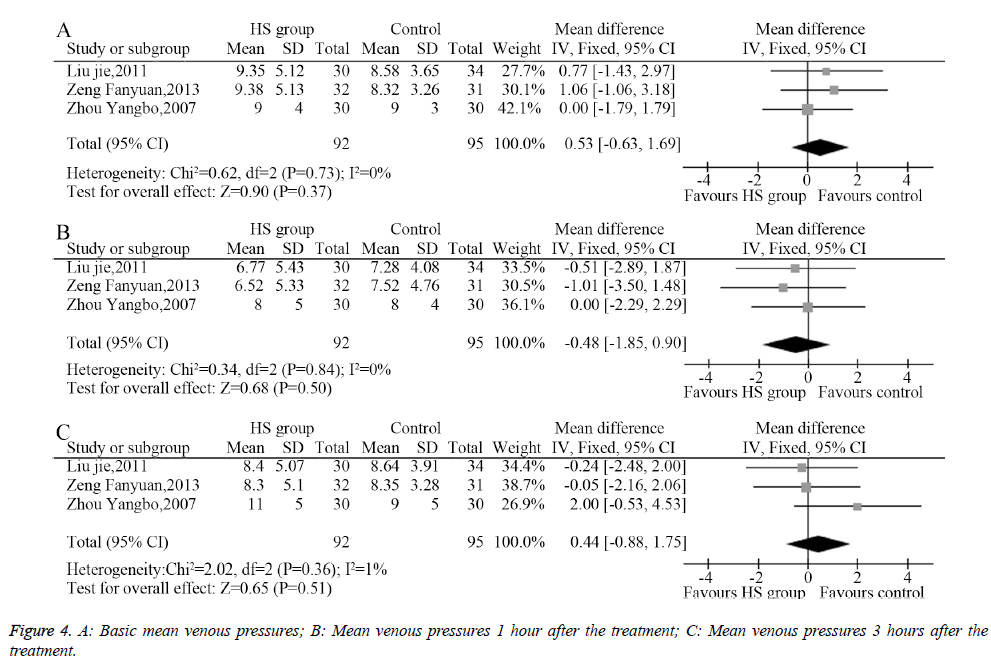
Three studies described the mean venous pressure and measured the mean venous pressure 1 and 3 hours after the treatment; the heterogeneity analysis provided suitable models for the combination of the included trials. The meta-analysis showed that the pretreatment mean venous pressures showed no significant differences between the HS group and the control group (MD=0.53, 95% CI: -0.63-1.69, P=0.37, Figure 4A). Moreover, the mean venous pressure 1 hour after the treatment (MD=-0.48, 95% CI: -1.85-0.90, P=0.50, Figure 4B) and 3 hours after the treatment showed no significant difference between groups (MD=0.44, 95% CI: -0.88-1.75, P=0.51, Figure 4C).
Analysis of publication bias
RevMan 5.3 software was used to generate a funnel plot (Figure 5) in which most points were within the scope of the inverted funnel, showing substantial symmetry located on both sides of the baseline, indicating no evidence of publication bias.
Discussion
The purpose of this study was to evaluate the effectiveness of HS in treating Chinese mainland populations with HIP, and the meta-analysis showed that in Chinese mainland populations, the 1 and 3 hour after treatment intracranial pressure, mean arterial pressure, and mean venous pressure between the HS treatment and other measures had no significant difference, indicating that HS had the same effectiveness in reducing IP in Chinese mainland populations as other measures, and no difference was found in any aspect of hemodynamic changes. This result is different from that of Kamel et al. [9] who found that HS was more effective in reducing IP than other treatments such as mannitol, but the discrepancy might be related to the concentration of HS. In Kamel’s meta-analysis [9], the doses of mannitol and HS used the same mmol osmotic pressure as the standard, but the clinical trials included in the current analysis did not limit the mmol osmotic pressure, but instead used one fixed HS concentration. In addition, the populations in these two studies were different, and different populations might have different sensitivities to sodium chloride; however, the results of these two studies showed that HS could effectively reduce cerebral edema and decrease intracranial pressure, so it could be used as a conventional treatment regimen for clinically reducing intracranial hypertension.
The mechanisms whereby HS reduces cerebral edema and HIP are still not fully understood; according to animal experiments and clinical trials, its mechanisms might be related to the following effects: osmotic dehydration; cerebral perfusion improvement, relieving the inflammations after brain injuries, or promoting the recycling of GLU neurotransmitters. The higher the osmotic reflection coefficient (ORC) of a substance, the less easily it passes through the blood-brain barrier [18,19]. The ORC of common sodium chloride, mannitol, and glycerol are 1.00, 0.90, and 0.59, respectively, and that of sodium chloride is higher than the other two, indicating that sodium chloride more easily passes through the blood-brain barrier, where it can establish osmotic gradients between blood vessels and tissues; therefore, water can enter the capillaries from the cells and tissue spaces, thus causing the amount of water in the brain to reduced, also reducing cerebral edema and IP.
After craniocerebral injuries, the hemorheological changes happen synchronically with brain edema, and HS can reduce the edema of vascular endothelial cells and red blood cells, thus increasing the inner diameter of blood vessels, increasing the deformability of red blood cells, and improving cerebral blood flow in the ischemic area [20]. It could also dilute the blood, reduce the blood viscosity, and improve cerebral perfusion, thus reducing cerebral edema, decreasing IP, and preventing secondary brain injuries [21]. The osmotic gradients generated after HSIVGTT could rapidly transfer the interstitial fluid and intracellular fluid into blood vessels and expand the blood volume. This hypertonic status could also shrink the swollen endothelial cells, reduce the capillary inner diameters back to normal, clear the microcirculation, reverse hemorrhagic shock, reduce anteroinferior cardiac loads, and improve tissue perfusion [22].
Brain injuries can lead to acute inflammatory responses, and the neutrophils gather at the injured sites, resulting in peroxidase and protease mediated cell necrosis. The activated leukocytes can also cause tissue edema. HS can regulate the immune system, inhibit the chemotaxis and adhesion of neutrophils, and inhibit the expression of CD11b; it can also inhibit the expression of proinflammatory cytokines (such as tumor necrosis factor-α), promote the expression of antiinflammatory factors (such as a leukocyte IL-10), and adjust the balance of local inflammatory responses, thus reducing primary traumatic brain injuries that lead to secondary injuries [23,24]. Brain injuries often cause the massive release of excitatory GLU, thus excessively activating its receptors and causing excitatory neurotoxicity, as well as further injuries to nerve cells. HS can restore the extracellular Na+ concentration, prompt the reuptake of GLU, and reduce extracellular GLU concentration, thus reducing the excitotoxic effects [25].
We believe that HS is safe and effective in preventing cerebral edema and treating HIP caused by various traumas. Studies have shown that HS not only exhibits superior IP reducing effects than mannitol but also these effects last longer and act faster. Meanwhile, it has no negative impact on the cerebral perfusion pressure; after considering the situation of systemic hemodynamics and electrolytes, HS could be considered as one selectable dehydrating agent. However, due to the limited number of relevant RCTs currently available, comparisons about central pontine demyelination, renal function, and nerve function scoring still lacked convincing data; the ideal concentration, maintenance time, and medication mode of HS are not very clear yet, and these all depend on conducting highquality large-scale randomized controlled clinical trials. Meanwhile, the clinical trial registration system should be further standardized, and investigators should use corresponding standardized and unified testing procedures to integrate clinical research resources as efficiently as possible, and therefore provide more reliable data for evidence-based clinical treatments.
The present study had several limitations: 1) only published studies were included, although the literature was rigorously searched and screened, trials with negative results are not easily published; meetings and abstracts were also excluded, so the included trials were not comprehensive. 2) The total number of the cases included was small, so it might not be able to represent the overall situation in the Chinese mainland. 3) The 10 included studies lacked any description of their blinding methodology, as well as unclear descriptions of how they concealed randomization, so the quality of the studies was relatively low, and low-quality studies often lead to the overevaluation of drug effectiveness; other studies have shown that low-quality studies can underestimate or overestimate the effectiveness of a drug [26,27]. 4) This study only investigated the effect of HS on intracranial pressure, mean arterial pressure, and mean venous pressure, and did not explore the differences caused by different HS concentrations, or the adverse reactions to different treatments. Therefore, we need more high-quality large-scale randomized controlled clinical trials to more comprehensively assess the efficacies and safety of HS in reducing IP, thus providing reliable evidence for its clinical applications.
Acknowledgements
This work was supported by grants from Key project of Jining Medical University (JY2013KJ047).
References
- Kojic B, Burina A, Hodzic R, Pasic Z, Sinanovic O. Risk factors impact on the long-term survival after hemorrhagic stroke. Med Arh 2009;63:203-206.
- Wong JM, Panchmatia JR, Ziewacz JE, Bader AM, Dunn IF, Laws ER, Gawande AA. Patterns in neurosurgical adverse events: intracranial neoplasm surgery. Neurosurg Focus 2012;33:E16.
- Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol Cell Physiol 2000;278:C144-C153.
- Rabinstein AA. Treatment of cerebral edema. Neurologist 2006;12:59-73.
- Deng YY, Shen FC, Xie D, Han QP, Fang M, Chen CB, Zeng HK. Progress in Drug Treatment of Cerebral Edema. Mini Rev Med Chem 2016;16:917-925.
- Li Q, Chen H, Hao JJ, Yin NN, Xu M, Zhou JX. Agreement of measured and calculated serum osmolality during the infusion of mannitol or hypertonic saline in patients after craniotomy: a prospective, double-blinded, randomised controlled trial. BMC Anesthesiol 2015;15:138.
- Kheirbek T, Pascual JL. Hypertonic saline for the treatment of intracranial hypertension. Curr Neurol Neurosci Rep 2014;14:482.
- Huang LQ, Zhu GF, Deng YY, Jiang WQ, Fang M, Chen CB, Cao W, Wen MY, Han YL, Zeng HK. Hypertonic saline alleviates cerebral edema by inhibiting microglia-derived TNF-α and IL-1β-induced Na-K-Cl Cotransporter up-regulation. J Neuroinflammation 2014;11:102.
- Kamel H, Navi BB, Nakagawa K, Hemphill JC 3rd, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med 2011;39:554-559.
- Guan Y, Shen F, Pan Q, Liu NT. Comparison the treatment of 7.5%hypertonic saline with 20% mannitol in patients with intracranial hypertension. J Qiqihar Medical College 2009;30:261-262.
- Lei M, Guan ZL, Yang GS. Hypertonic saline treatment of traumatic intracranial hypertension. Chinese J Medicinal Guide 2010;12:982-983.
- Liu J, Li G, Zhang SY, Gao ZG, Song HF, Liu HL, Zhang SQ. Clinical research of hypertonic saline in treating large-area cerebral infarction-induced secondary encephaledema and intracranial hypertension. Shandong Medical J 2011;51:100-102.
- Mo L, Xue N. 69 cases of encephaledema treated with combination of Furosemide and mannitol. China Pharmaceuticals 2014;23:88-90.
- Pan JH, Cheng C, Chen J, Chen Z, Hang Q. Cinical observation of 40 hypertonic sodium chloride hydroxyethyl starch injection in the treatment of traumatic brain injury. Heilongjiang Medical J 2013;37:677-679.
- Yang ZG, Gong Y, Zhou MH. Clinical observation of hypertonic sodium chloride hydroxyethyl starch 40 injection in treating traumatic brain injury. Modern Medical J 2011;39:149-152.
- Zeng FY, Yang JX, Zhou Y, Deng ZB, Hu MJ. Efficacy comparison of hypertonic saline and mannitol in treating Intracranial Hypertension. Chinese J Preatical Neruous Diseases 2013;16:90-91.
- Zhou YB, Jiang YG, Zhang LY, Li MH, Xiang J. Efficacy analysis of hypertonic saline in treating severe craniocerebral trauma combined with early shock. JTraumatic Surg 2007;9:211-214.
- Ziai WC, Toung TJ, Bhardwaj A. Hypertonic saline: first-line therapy for cerebral edema? J Neurol Sci 2007;261:157-166.
- Diringer MN. New trends in hyperosmolar therapy? Curr Opin Crit Care 2013;19:77-82.
- Scalfani MT, Dhar R, Zazulia AR, Videen TO, Diringer MN. Effect of osmotic agents on regional cerebral blood flow in traumatic brain injury. J Crit Care 2012;27:526.e7-12.
- Fink ME. Osmotherapy for intracranial hypertension: mannitol versus hypertonic saline. Continuum (Minneap Minn) 2012;18:640-654.
- Qureshi AI, Suarez JI. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit Care Med 2000;28:3301-3313.
- Thongrong C, Kong N, Govindarajan B, Allen D, Mendel E, Bergese SD. Current purpose and practice of hypertonic saline in neurosurgery: a review of the literature. World Neurosurg 2014;82:1307-1318.
- Hirsh M, Dyugovskaya L, Bashenko Y, Krausz MM. Reduced rate of bacterial translocation and improved variables of natural killer cell and T-cell activity in rats surviving controlled hemorrhagic shock and treated with hypertonic saline. Crit Care Med 2002;30:861-867.
- Charalambous MP, Swoboda SM, Lipsett PA. Perioperative hypertonic saline may reduce postoperative infections and lower mortality rates. Surg Infect (Larchmt) 2008;9:67-74.
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-613.
- Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ 1998;317:1185-1190.