Review Paper - Journal of Molecular Oncology Research (2018) Volume 2, Issue 3
A key receptor in apoptosis: Toll-like receptor 3 (TLR3).
Asuman Deveci Ozkan1, Humeyra Nur Kaleli2 and Suleyman Kaleli1*
1Faculty of Medicine, Department of Medical Biology, Sakarya University, Turkey
2Faculty of Engineering and Natural Sciences, Department of Molecular Biology, Genetics and Bioengineering, Sabanci University, Turkey
- *Corresponding Author:
- Suleyman Kaleli
Faculty of Medicine, Department of Medical Biology
Sakarya University, Korucuk Campus, Sakarya, Turkey
E-mail: skaleli@sakarya.edu.tr
Accepted date: September 25, 2018
Citation: Ozkan AD, Kaleli HN, Kaleli S. A key receptor in apoptosis: Toll-like receptor 3 (TLR3) J Mol Oncol Res. 2018;2(3):63-67.
DOI: 10.35841/molecular-oncology.2.3.63-67
Visit for more related articles at Journal of Molecular Oncology ResearchAbstract
The mammals have two types of immune system as innate and acquired immunity. The innate immune system initiates inflammatory response as well as phagocytosis to microbial attack, thus forming the organism's first line of protection. This inflammatory response is the result of induction of pattern recognition receptors (PRRs) which is inherited in the organism. TLRs are the best defined receptors among the PRRs. TLR3 is activated by double-stranded RNA/polyinosinic–polycytidylic acid (dsRNA, poly (I:C)). TRIF protein plays a role as an adaptor protein in TLR3 signaling pathway. The dsRNA-induced apoptosis by the ligand of TLR3 requires activation of RIP1, caspase-3 and caspase-8. While TLR3 signaling can contribute to the eradication of tumors with the RIP-1/FADD pathway, it increases the activation of IFN-α, IFN-β and NK cells and it also leads to the formation of pro-angiogenic factors that contribute to tumor progression through NF-κB activation. TLR3 stimulation with dsRNA is considered directly promoting tumor cell apoptosis in many types of cancer such as breast, melanoma, prostate, cervical, colon and hepatocellular carcinoma. It is known that poly (I:C) is directly cause apoptosis in cancer cells by caspase-dependent pathway. It has also been shown that certain NF-κB proteins are required for TLR3-mediated apoptosis. Because of its high toxicity, Poly (I:C) cannot be used in chemotherapy. Studies have shown that dsRNA induces apoptosis by caspase-dependent manner but there is no clear evidence of how TLR3 plays a role in dsRNA-mediated apoptosis. The molecular mechanism of TLR3-mediated apoptosis needs to be fully understood.
Keywords
TLR3, Apoptosis, TRIF, MyD88
Abbreviations
ALR: AIM2-Like Receptor; CLR: C-Type Lectin Receptor; dsRNA: Double Strand RNA; FADD: Fas-Associated Death Domain; IKK: I-KappaB Kinase; IL-1R: Interleukin-1 Receptor; IFN: Interferon; IRF: Interferon Regulatory Factor; MyD88: Myelin and Lymphocyte Protein MAL Myeloid Differentiation Primary Response 88; NK cell: Natural Killer Cell; NLR: NOD-Like Receptor; NF-κB: Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells; PAMP: Pathogen-Associated Molecular Pattern; PRR: Pattern Recognition Receptor; PKC: Protein Kinase C; RIP: Receptor Interacting Protein 1; RLR: RIG-I-Like Receptor; SARM: Sterile Alpha and TIR Motif-Containing Protein; TANK: TANK Binding Kinase TBK TRAF Family Member Associated NF-κB Activator; TIRAP: TIR Domain Containing Adapter Inducing Interferon-β TRIF TIR Domain Containing Adaptor Protein; TRAM: TRIF-Related Adaptor Molecule; TICAM1: Toll/IL-1R Domain Containing Adaptor Molecule 1; TLR: Toll-Like Receptor.
Introduction
The mammals have two types of immune system as innate and acquired immunity. The innate immune system was thought to have a non-complex structure up to the mid-1990s, but it was later revealed that it has a complex mechanism at an advanced level, like the acquired immunity [1]. The innate immune system initiates inflammatory response as well as phagocytosis to microbial attack, thus forming the organism's first line of protection. This inflammatory response is the result of induction of pattern recognition receptors (PRRs) which is inherited in the organism. PRRs recognize both microbial pathogen-associated molecular patterns (PAMPs) and immunoreactions in substances secreted by the organism itself, such as in stress, tissue damage, and necrotic cell death [2]. In addition, PRRs are also involved in important processes such as regulation of apoptosis, DNA repair, autophagy and angiogenesis [3]. PRRs consist of five different receptor families according to the homology of the protein domains, as Toll-like receptor (TLR), NOD-like receptors (NLR), C-type lectin receptors (CLR), RIG-I-like receptors (RLR) and AIM2- like receptors (ALR). TLRs are the best defined within these receptors [1]. TLRs have been conserved in the evolutionary process from the worm species Caenorhabditis elegans to mammalian [4-7]. Toll was initially identified as an essential gene in the development of the embryonal dorsoventral polarity of Drosophila. In studies conducted in the following years, it has been determined that these genetically silenced flies are killed by fungal infections and this gene also has antifungal effect at the same time [8].
In human 10 different TLRs (TLR1-TLR10) and in mice 12 different TLRs (TLR1-TLR9, TLR11-TLR13) have been defined. TLRs are localized to the cell surface or to intracellular compartments such as endosomes, lysosomes, and endolysosomes. TLR3, TLR8, TLR9, TLR11, TLR12 and TLR13 are found in the endosomes while TLR1, TLR2, TLR4, TLR5, TLR6 and TLR10 are localized on the cell surface. TLRs can be expressed in the immune system cells such as macrophages and dendritic cells as well as the cells other than the immune system such as fibroblast, epithelial and tumor cells [9,10].
Different TLRs show specificity while recognizing their ligands. TLR3 is activated by double-stranded RNA/ polyinosinic–polycytidylic acid (poly (I:C)) [11,12]. Adapter proteins (MyD88, TIRAP/MAL, TRIF, TRAM and SARM) are required for transmit the signal initiated by members of the TLRs and IL-1R family. TRIF protein plays a role as an adaptor protein in TLR3 signaling pathway [13]. These adapter proteins interact each other for activate transcription factors (NF-κB, IRF1, IRF3, IRF7) and interferon-gamma [14].
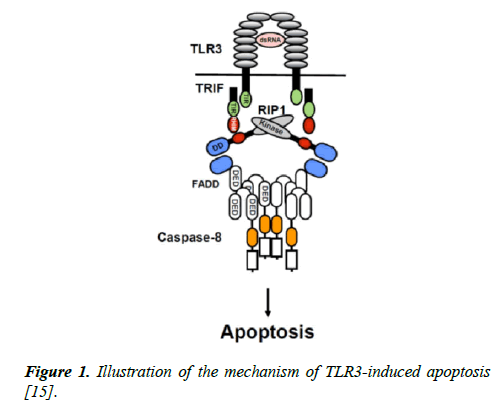
There is an association between TLR signaling and apoptosis induction in cancer cells [16]. The most effective TLRmediated cell death is apoptosis which is leading to programmed cell death including the activation of catabolic enzymes and the destruction of cell organelles [17]. The mechanism of TLR3-triggered apoptosis is shown in Figure 1. TLR3 induction by dsRNA induces the formation of caspase-8- activating complex including caspase8/FADD/RIP1/TRIF and TLR3. TLR3 has a TIR domain which binds to the adaptor TRIF via TIR domain. After interaction with TRIF, RIP1 recruits FADD through Death Domain (DD) and FADD recruits caspase-8 through Death Effector Domain (DED) (Figure 1) [15].
Figure 1: Illustration of the mechanism of TLR3-induced apoptosis [15].
TLR3 does not have the death domain which is found at death receptors and it is unclear that the dsRNA-triggered apoptosis basis is via the caspase-8 pathway. In addition, recent studies indicate that TRIF and RIP1 are essential for dsRNA-triggered apoptosis through the caspase-8 pathway [18-20]. TLR3 signal is regulated by binding of the adaptor-inducing IFN-β (TRIF) adapter protein which induces the TIR domain of the TLR3 receptor. This results in the activation of NF-κB and IRF3 transcription factors and the synthesis of interferons at the end of the TLR3 signaling pathway [21]. TRIF also demonstrates pro-apoptotic activity and demonstrates that the TLR3 signal triggers the apoptosis [22].
TLR3 is the most specific receptor among TLRs. The inflammatory response and apoptosis can be induced by TLR3 signaling through TRIF adaptor protein rather than MyD88 [23]. For this purpose, the aim of this study was to discuss the role of TLR3 in tumor cells and the relationship between apoptosis and the treatment of cancer.
TLR3 expressed in tumor cells
TLRs are not only expressed on natural immune system cells; but also expressed in non-immune cells involving the cancer cells [24-26]. It is known that cancer progression is associated with both TLRs in both the immunocyte cells and tumors [27]. TLRs serve as basic sensor molecules in the immune system that detect tumor-derived antigens and stimulate the primer anti-tumor immune response [28]. Activated TLRs trigger signaling pathways by triggering cytotoxic activity in effector cells and cause lysis of tumor cells [29]. Removal of tumor cells in time via TLRs can also prevent the formation of inflammatory tumor microcirculation cells [30]. However, it has been reported that different TLRs have opposite effects in cancer development [31]. In some cases, tumor cells activate some TLRs that are immunosuppressive, leading to a decrease in the number of cytotoxic cells and an increase in pro-inflammatory factors [32].
There are many studies in the literature showing that TLRs are expressed at high levels in tumor cells [33-37] such as in colon cancer cells TLR2, TLR3 and TLR4 [38,39], in ovarian cancer cells TLR2, TLR3, TLR4 and TLR5 [40,41], in prostate and colorectal cancer cells TLR3, TLR4 and TLR9 expression has been detected [42].
TRIF adaptor protein
TRIF is also called as Toll/IL-1R domain containing adaptor molecule 1 (TICAM1]. In TLR3 and TLR4 signaling pathway, TRIF acts as a first adaptor protein. When IRF-3 is activated by TRIF, two protein kinases are involved; TRAF family member-associated NF-κB activator (TANK) binding kinase (TBK1) and inducible IkappaB kinase (IKKi). Both of the kinases phosphorylate IRF-7, which is involved in the production of type I IFNs [4]. TRIF has 712 amino acids and considered as a large protein. While overexpression of TRIF with MyD88 and MAL activates NF-κB-dependent pathway in HEK-293 cells, overexpression of TRIF alone initiated the induction of molecules that release IFN-β. In TLR3 and TLR4 mediated IFN-β production as well as IFN-inducible gene expression disorders were seen in TRIF knockout mice [14,43].
TLR3 signaling pathway and apoptosis
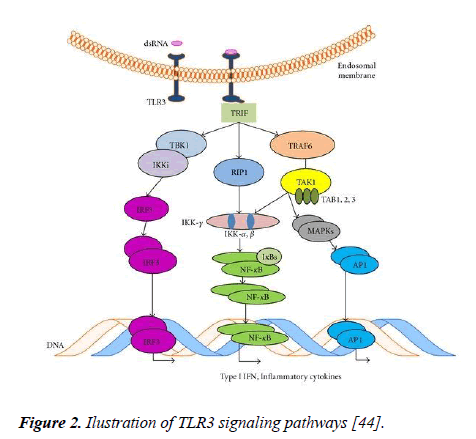
Type I interferon production is necessary for cell death initiated by TLR3 agonists, but not enough for apoptosis. NFkB, p65 and extrinsic caspases are also necessary for apoptosis initiated by TLR3. According to Muccioli et al. [14], an illustration of the TLR3 signaling pathway was shown in Figure 2.
Figure 2: Ilustration of TLR3 signaling pathways [44].
The largest adapter among the TLR adapter proteins is the TRIF and can initiate various signaling pathways [44]. NF-κB is activated by TLR3 signaling in a MyD88-independent pathway. After stimulation with the ligand, the TLR3 signal initiates a downstream signaling pathway with the TRIF adapter protein (Figure 2) which activates the NF-kB and IRF3 transcription factors with JNK and p38 pathways [45].
TRIF has an N-terminal region that allows IRF3 and NF-κB activation by accumulation of TNF receptor-associated factor (TRAF) proteins and also has an C-terminal binding site, that allows JNK and p38 activation which results in apoptosis and autophagy by accumulation of receptor interacting protein (RIP-1) and Fas-associated death domain (FADD). While TLR3 signaling can contribute to the eradication of tumors with the RIP-1/FADD pathway, increases the activation of IFN-α, IFN-β and NK cells and it also leads to the formation of pro-angiogenic factors that contribute to tumor progression through NF-κB activation [44].
TLR3-mediated apoptosis in various cells
TLR3 stimulation with dsRNA is considered directly promote tumor cell apoptosis in many types of cancer such as breast [18], melanoma [46], prostate [47], cervical [48], colon [48] and hepatocellular carcinoma [49]. TLR3- dependent apoptosis of endothelial cells triggered by dsRNA is dependent on the extrinsic pathway [50], dsRNA induced TLR3-dependent apoptosis involves the pro-apoptotic signaling by TRIF in melanoma [50], IRF-3 pathway in pancreatic cells [51], protein kinase C (PKC)-dependent mechanism in prostate cancer cells [47,52] and TRIF and type I IFN autocrine signaling in breast cancer cells [53]. TLR3-dependent apoptosis includes caspase activation and extrinsic and intrinsic apoptotic pathways [54]. TLRs can also directly affect cancer cells. When TLR3 stimulated with dsRNA in breast cancer, cells are beginning to produce autocrine type I IFN which is regulating TLR3- medaited cell death [18,55]. Likewise an atypical caspase-8- containing complex which induces apoptotic pathways is increasing when TLR3 stimulated with dsRNA in lung cancer [8].
TLR3 in cancer treatment
Recent studies have shown that TLR3 can be a potential therapeutic agent in many cancer types. Human pharyngeal cancer cell lines and oral squamous cell carcinoma cell lines express and activate TLR3 receptor and this signaling enhance apoptosis in tumor cells [25,55]. Interestingly fighting cancer by targeting TLR3 emerges as new strategies. Researchers have designed a new immunotherapeutic method based on cancer vaccination. In this study, they generated poly (I:C)- DOTAP liposome complex nanoparticles, which enhance poly (I:C) cellular penetration, increased the anti-tumoral activity of TLR3 signal in BMDCs [56]. It is known that poly (I:C) is directly cause apoptosis in cancer cells by caspase-dependent pathway [18]. It has also been shown that certain NF-κB proteins are required for TLR3-mediated apoptosis. Because of its high toxicity, Poly (I:C) cannot be used in chemotherapy [18,45]. The anti-cancer effect of a single TLR agonist is need to quantification and when we consider the side effects, it may be premature for clinical application in cancer treatment. In a study, it was shown that TLR3 has the two-way tumor effects which make TLR3 a risky therapeutic agent. The TLR3 agonist poly A:U is therapeutically effective in many types of cancer, but it decreases the risk of metastasis in patients with TLR3- positive breast cancer, not in TLR3-negative breast cancer patients [57].
Discussion
Apoptosis can be induced as a result of stimulation of TLRs using with MyD88, TRIF, or both. This suggests that apoptosis may be activated by MyD88 and TRIF in an independent manner, and that the intersection of signaling pathways initiated by these two adapter proteins may be resulted by apoptosis. The role of TLRs in cancer cells differs according to the type of cancer and its origin. Therefore, more studies and further evidence are needed to clinically use TLR agonists as therapeutic agents. Studies have shown that dsRNA induces apoptosis by caspase-dependent manner but there is no clear evidence of how TLR3 plays a role in dsRNA-mediated apoptosis. The molecular mechanism of TLR3-mediated apoptosis needs to be fully understood. Thus, the signal pathway initiated by the TLR3 signal as a pathway outside of death receptors of apoptosis plays a key role in the regulation of apoptosis and perhaps as a potentially therapeutic agent. Considering this situation, recently, the role of TLRs in cancer has been the focus of researchers.
References
- Kumar H, Kawai T, Akira S. Pathogen Recognition by the Innate Immune System. Int Rev Immunol. 2011;30(1):16-34.
- Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013;13(8):551-65.
- Kutikhin AG, Yuzhalin AE, Tsitiko EA, et al. Editorial: Pattern Recognition Receptors and Cancer. Front Immunol. 2014;5:343.
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499-511.
- Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430(6996):257-63.
- Janeway CA Jr, Medzhitov R. Innate Immune Recognition. Annu Rev Immunol. 2002;20:197-216.
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426(6962):33-8.
- Lemaitre B, Nicolas E, Michaut L, et al. The Dorsoventral Regulatory Gene Cassette spätzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell. 1996;86(6):973-83.
- Celhar T, Magalhaes R, Fairhurst AM. TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol Res. 2012;53(1-3):58-77.
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373-84.
- Heil F, Hemmi H, Hochrein H, et al. Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8. Science. 2004;303(5663):1526-9.
- Hasan U, Chaffois C, Gaillard C, et al. Human TLR10 Is a Functional Receptor, Expressed by B Cells and Plasmacytoid Dendritic Cells, Which Activates Gene Transcription through MyD88. J Immunol. 2005;174(5):2942-50.
- Mishina T, Watanabe H, Araki H et al. Epidemiological study of prostatic cancer by matched-pair analysis. Prostate. 1985;6(4):423-36.
- Narayanan KB, Park HH. Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis. 2015;20(2):196-209.
- Shi M, Chen X, Ye K, et al. Application potential of toll-like receptors in cancer immunotherapy: Systematic review. Medicine. 2016;95(25):e3951.
- Bertin S, Carle PV. Autophagy and toll-like receptors: A new link in cancer cells. Autophagy. 2008;4(8):1086-9.
- Ramakrishnan R, Gabrilovich DI. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol Immunother. 2013;62(3):405-10.
- Salaun B, Coste I, Rissoan MC, et al. TLR3 Can Directly Trigger Apoptosis in Human Cancer Cells. The J Immunol. 2006;176(8):4894-901.
- Weber A, Kirejczyk Z, Besch R, et al. Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ. 2009;17(6):942-51.
- Feoktistova M, Geserick P, Kellert B, et al. cIAPs Block Ripoptosome Formation, a RIP1/Caspase-8 Containing Intracellular Cell Death Complex Differentially Regulated by cFLIP Isoforms. Mol Cell. 2011;43(3):449-63.
- Yamamoto M, Sato S, Hemmi H, et al. Role of Adaptor TRIF in the MyD88-Independent Toll-Like Receptor Signaling Pathway. Science. 2003;301(5633):640-3.
- Heylbroeck C, Balachandran S, Servant MJ, et al. The IRF-3 Transcription Factor Mediates Sendai Virus-Induced Apoptosis. J Virol. 2000;74(8):3781-92.
- Khvalevsky ZE, Abramovitch R, Barash H, et al. Toll-like receptor 3 signaling attenuates liver regeneration. Hepatology. 2009;50(1):198-206.
- Shi M, Yao Y, Han F, et al. MAP1S Controls Breast Cancer Cell TLR5 Signaling Pathway and Promotes TLR5 Signaling-based Tumor Suppression. PLoS One. 2014;9(1):e86839.
- Shatz M, Menendez D, Resnick MA. The human TLR innate immune gene family is differentially influenced by DNA stress and p53 status in cancer cells. Cancer Res. 2012;72(16):3948-57.
- Ridnour LA, Cheng RYS, Switzer CH, et al. Molecular Pathways: Toll-like Receptors in the Tumor Microenvironment—Poor Prognosis or New Therapeutic Opportunity. Clin Cancer Res. 2013;19(6):1340-6.
- DeCarlo CA, Rosa B, Jackson R, et al. Toll-Like Receptor Transcriptome in the HPV-Positive Cervical Cancer Microenvironment. Clin Dev Immunol. 2012;2012:785825.
- Ito T, Amakawa R, Fukuhara S. Roles of toll-like receptors in natural interferon-producing cells as sensors in immune surveillance. Hum Immunol. 2002;63(12):1120-5.
- Kalb ML, Glaser A, Stary G, et al. TRAIL(+)Human Plasmacytoid Dendritic Cells Kill Tumor Cells In Vitro: Mechanisms of Imiquimod- and IFN-α–Mediated Antitumor Reactivity. J Immunol. 2012;188(4):1583-91.
- Pisetsky DS, Gauley J, Ullal AJ. HMGB1 and Microparticles as Mediators of the Immune Response to Cell Death. Antioxid Redox Signal. 2011;15(8):2209-19.
- Cai Z, Sanchez A, Shi Z, et al. Activation of Toll-Like Receptor 5 on Breast Cancer Cells by Flagellin Suppresses Cell Proliferation and Tumor Growth. Cancer Res. 2011;71(7):2466-75.
- Chen R, Alvero AB, Silasi DA, et al. Cancers take their Toll-the function and regulation of Toll-like receptors in cancer cells. Oncogene. 2008;27(2):225-33.
- Ribeiro RA, Wanderley CWS, Wong DVT, et al. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: insights into pathogenesis and therapeutic perspectives. Cancer Chemother Pharmacol. 2016;78(5):881-93.
- Prakash H, Nadella V, Singh S, et al. CD14/TLR4 priming potentially recalibrates and exerts anti-tumor efficacy in tumor associated macrophages in a mouse model of pancreatic carcinoma. Sci Rep. 2016;6:31490.
- Llitjos JF, Auffray C, Laurent AF, et al. Sepsis-induced expansion of granulocytic myeloid-derived suppressor cells promotes tumour growth through Toll-like receptor 4. J Pathol. 2016;239(4):473-83.
- Kuo WT, Lee TC, Yu LCH. Eritoran Suppresses Colon Cancer by Altering a Functional Balance in Toll-like Receptors That Bind Lipopolysaccharide. Cancer Res. 2016;76(16):4684-95.
- Curtin JF, Liu N, Candolfi M, et al. HMGB1 Mediates Endogenous TLR2 Activation and Brain Tumor Regression. PLoS Med. 2009;6(1):e10.
- Furrie E, Macfarlane S, Thomson G, et al. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115(4):565-74.
- Fukata M, Chen A, Vamadevan AS, et al. Toll-like receptor-4 (TLR4) promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133(6):1869-81.
- Zhou M, McFarland-Mancini MM, Funk HM, et al. Toll-like receptor expression in normal ovary and ovarian tumors. Cancer Immunol Immunother. 2009;58(9):1375-85.
- Kelly MG, Alvero AB, Chen R, et al. TLR-4 Signaling Promotes Tumor Growth and Paclitaxel Chemoresistance in Ovarian Cancer. Cancer Res. 2006;66(7):3859-68.
- Niedzielska I, Orawczyk T, Ziaja K, et al. Toll-like receptors and the tendency of normal mucous membrane to transform to polyp or colorectal cancer. J Physiol Pharmacol. 2009;60(Suppl 1):65-71.
- Yamamoto M, Sato S, Hemmi H, et al. TRAM is specifically involved in the Toll-like receptor 4–mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4(11):1144-50.
- Muccioli M, Sprague L, Nandigam H, et al. Toll-Like Receptors as Novel Therapeutic Targets for Ovarian Cancer. ISRN Oncol. 2012;2012:642141.
- Seya T, Matsumoto M. The extrinsic RNA-sensing pathway for adjuvant immunotherapy of cancer. Cancer Immunol, Immunother. 2009;58(8):1175-84.
- Salaun B, Lebecque S, Matikainen S, et al. Toll-like Receptor 3 Expressed by Melanoma Cells as a Target for Therapy? Clin Cancer Res. 2007;13(15):4565-74.
- Paone A, Starace D, Galli R, et al. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-α-dependent mechanism. Carcinogenesis. 2008;29(7):1334-42.
- Jiang Q, Wei H, Tian Z. Poly I:C enhances cycloheximide-induced apoptosis of tumor cells through TLR3 pathway. BMC Cancer. 2008;8:12.
- Khvalevsky E, Rivkin L, Rachmilewitz J, et al. TLR3 signaling in a hepatoma cell line is skewed towards apoptosis. J Cell Biochem. 2007;100(5):1301-12.
- Pries R, Hogrefe L, Xie L, et al. Induction of c-Myc-dependent cell proliferation through toll-like receptor 3 in head and neck cancer. Int J Mol Med. 2008;21(2):209-15.
- He J-F, Jia W-H, Fan Q, et al. Genetic polymorphisms of TLR3 are associated with Nasopharyngeal carcinoma risk in Cantonese population. BMC Cancer. 2007;7:194.
- He W, Liu Q, Wang L, et al. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007;44(11):2850-9.
- Satoh K, Kaneko K, Hirota M, et al. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92(2):271-8.
- Kaiser WJ, L Kaufman J, Offermann M. IFN-α Sensitizes Human Umbilical Vein Endothelial Cells to Apoptosis Induced by Double-Stranded RNA. J Immunol. 2004;172(3):1699-710.
- Estornes Y, Toscano F, Virard F, et al. dsRNA induces apoptosis through an atypical death complex associating TLR3 to caspase-8. Cell Death Differ. 2012;19(9):1482-94.
- Wang C, Zhuang Y, Zhang Y, et al. Toll-like receptor 3 agonist complexed with cationic liposome augments vaccine-elicited antitumor immunity by enhancing TLR3–IRF3 signaling and type I interferons in dendritic cells. Vaccine. 2012;30(32):4790-9.
- Salaun B, Zitvogel L, Paturel AC, et al. TLR3 as a Biomarker for the Therapeutic Efficacy of Double-stranded RNA in Breast Cancer. Cancer Res. 2011;71(5):1607-14.