- Biomedical Research (2006) Volume 17, Issue 2
A Comparative Study of the Chronological Appearance of Adenohypophysial Cells in Vertebrates with Emphasis on the Japanese Soft-Shelled Turtle (Pelodiscus sinensis japonicus)
Tsuyoshi Saga and Koh-ichi YamakiDepartment of Anatomy, Kurume University, School of Medicine, 67, Asahi-machi, Kurume 830-0011, JAPAN
Accepted date: April 17, 2006
Abstract
We previously reported the chronological appearance of adenohypophysial cells in several vertebrates using an immunocytochemical technique. The present study investigated the chronological appearance of adenohypophysial cells in the freshwater Japanese soft-shelled turtle (Pelodiscus sinensis japonicus). The results were compared with those of the sea turtle, as well as other vertebrates which were studied by us and other researchers. In adult Japanese soft-shelled turtle adenohypophysis, six or seven types of secretory cells were distinguished; prolactin (PRL), growth hormone (GH), thyroid stimulating hormone (TSH), gonadotropic hormones (LH and FSH), adrenocorticotropic hormone (ACTH), and melanophore stimulating hormone (MSH). Chronologically, on five days after spawning, the first cells to appear were TSH, LH, ACTH and MSH cells. Subsequently, 13 days after spawning, a few FSH cells were observed; 22 days after spawning, GH and PRL cells appeared. These results, relating to the localization patterns and chronological order of appearance of the adenohypophysial cells, were similar to the sea turtle. Moreover, we demonstrate the chronological appearance of six other animal species (rainbow trout, Nile tilapia, Ayu, Japanese whiting, clouded salamander and golden hamster). A comparative study of other animals investigated by different researchers showed that each animal had a species-specific order of the appearance of the adenohypophysial cells and suggested the order was closely related to developmental style, spawning and environment of each animal. Hormones appearing during the early developmental stage may be required to survive in each species-specific environment.
Keywords
Adenohypophysis, Developmental biology, Cell differentiation Immunohistochemistry, Reptile, Turtle, Vertebrate, Comparative
Introduction
The pituitary is one of the most important endocrine organs in vertebrates. A number of studies have been performed on the cytophysiology of the pituitary gland of many species of animals by a variety of conventional staining methods, including immunocytochemical techniques (e.g. Doerr-Schott, [1,2]). In the adenohypophysis, seven to eight types of secretory cells have been distinguished, each of which produce different hormones; prolactin (PRL), growth hormone (GH), thyroid stimulating hormone (TSH), gonadotropic hormone (GTH; in salmonid fish and several other species, GTH-I and GTH-II in tetrapods), luteinizing hormone (LH) and follicle stimulating hormone (FSH)), adrenocorticotropic hormone (ACTH), melanophore stimulating hormone (MSH), and somatolactin (SL; only in fish). Recently, we studied embryological development of the adenohypophysial hormone producing cells in four species of teleosts: rainbow trout (Oncorhynchus mykiss irideus) [3], Nile tilapia (Oreochromis niloticus) [4], Ayu (Plecoglossus altivelis) [5], and Japanese whiting (Sillago japonica) [6]). Moreover, in the past, we reported on an amphibian; the Clouded salamander (Hynobius nebulosus) [7] and a mammal; the golden hamster (Mesocricetus auratus) [8]. As a result, we showed that each animal had a species-specific order of the appearance of the adeno-hypophysial cells, and suggested the order was related to developmental style, spawning and environment of the each animal. In other vertebrates, many studies have been reported on the chronological appearance of all or a part of adenohypophysial cells by using immunocytochemical staining methods. In the teleosts, coho salmon (Oncorhyn-chus kisutch) [9], sea bass (Dicentrarchus labrax) [10], chum salmon (Oncorhynchus keta) [11], and American shad (Alosa sapidissima) [12] were investigated. In amphibians, several species were used to investigate the morphogenesis of the pituitary; Xenopus laevis [13], Ambystoma gracile [14], Rana dalmatina [15]. In reptiles, the sea turtle (Caretta caretta) was studied [16]. In the aves, most investigations were performed using chickens [17,18,19]. In mammals, there have been reports concerning humans [20,21], rats [22-29], and hamsters [30,31,32]. In reptiles, only a single species of sea turtle had the ontogeny reported of the adenohypophysial cells by Pearson et al. [16]. To date, no studies have been made on the embryological development of the adenohypophysial hormone producing cells in the fresh water turtle. Therefore, in this study, we investigated the adult adenohypophysis and development of adenohypophysis in the Japanese soft-shelled turtle, Pelodiscus sinensis japonicus. This species inhabits fresh water and spawns under the ground near rivers, ponds and lakes and develops in fresh water. We compared the results of the Japanese soft-shelled turtle with those of the sea turtle [16]. Moreover, we complemented our previous results of the ontogeny of adenohypophysial cells with those of the six species of animals mentioned above [3-8]. Finally, these results were compared with the adenohypophysial development in other vertebrates.
Materials and Methods
Materials
Embryos and adults of the Japanese soft-shelled turtle, Pelodiscus sinensis japonicus, were provided by the Freshwater Laboratory of the Oita Institute of Marine Fisheries Science. Fertilized eggs were bred in laboratory conditions at a constant temperature (30°C). Embryos were sampled at various developmental stages from 5 days after spawning (about 35 days before hatching) to about 1 month after hatching. At each stage, at least six animals were sampled. Some adult turtles of both sexes were used as a reference for pituitary cytology.
Histological and cytological procedures
All animals were sacrificed by decapitation. The whole body or heads of small embryos and the pituitaries of young and adult turtles were fixed for 24 h in Bouin’s solution. Tissue samples were dehydrated through a series of solutions with increasing concentrations of ethanol. Tissues were embedded in paraffin, and serial sagittal sections of 5 μm were mounted on glass slides. The preparations of the pituitaries and heads, including the brain with pituitary, were stained with Haematoxylin-Eosin to permit observation of the general structure of the pituitary.
Primary Antibodies
The following primary antisera were used in this study: anti-human growth hormone (GH, diluted 1:2000), anti-chum salmon growth hormone (GH, diluted 1:2000, provided by Dr. H. Kawauchi, School of Fisheries, Kita-sato University, Iwate, Japan) [33,34]), anti-human prolactin (PRL, diluted 1:2000), Anti-chum salmon prolactin (PRL, diluted 1:4000, provided from Dr. Y. Nakai, School of Medicine, Showa University, Tokyo, Japan [33,35]), anti-beta subunit of human thyroid stimulating hormone (TSH, diluted 1:500), anti-beta subunit of human luteinizing hormone (LH 1:1000), anti-beta subunit of human follicle stimulating hormone (FSH 1:1000), and antiporcine adrenocorticotrophic hormone (ACTH 1:-500), anti α-melanophore stimulating hormone (α-MSH, diluted 1:300, provided by Dr. B. Baker, University of Bath, Bath, England [36]), anti-coho salmon gonadotropin (GTH) I beta subunit (diluted 1:1600) and anti-coho salmon GTH-II beta subunit (diluted 1:800) were provided from Dr. P. Swanson, School of Fisheries, University of Washington, Seattle, U.S.A. [37]. Anti-salmon somato-lactin (SL) (diluted 1:5000) was provided form Dr. H. Kawauchi, School of Fisheries, Kitasato University, Iwate, Japan.
Staining Procedures of Immunocytochemistry
Immunocytochemical staining was performed with a Histofine kit (Nichirei, Japan). In brief, sections were deparaffinized in xylene, hydrated in a graded ethanol series, and washed in phosphatebuffered saline (10mM sodium phosphate, 0.15 M sodium chloride, pH 7.5; PBS). All procedures were performed at room temperature, and incubations were performed in closed humid chambers. First, the tissue sections were incubated for 30 min in methanol containing 0.3% hydrogen peroxide to block endogenous peroxidase activities, and were subsequently washed in PBS. To reduce nonspecific staining caused by the biotinylated antirabbit IgG, sections were treated with normal goat serum for 30min, and then washed in PBS. Primary antisera were applied to the sections for 18 h, and biotinylated anti-rabbit IgG and peroxidase-conjugated streptavidin were applied for 1 h each. The final reactive products were visualized with 3,3’-diaminobenzi-dine tetrahydrochloride in 50 mM Tris-HCl buffer (pH 7.6) containing 0.003% hydrogen peroxide [38]. The sections were subsequently counterstained with haematoxylin, washed in running water, dehydrated through an increased ethanol series, and mounted in Diatex (Ab Wilh Becker, Sweden). Each of the above mentioned antibodies was applied to sections prepared from at least six animals at each developmental stage. To confirm the specificity of the immunocytochemical staining, the primary antiserum was replaced by PBS.
Results
In the Japanese soft-shelled turtle, morphologically, the adenohypophysis was divided into three regions; the pars tuberalis (PT), the pars distalis (PD) and pars intermedia (PI). The PT was located on the anterior-upper side of the PD and occupied with the median eminence the ventral side of the third ventricle. The PD occupied most of the central area of the adenohypophysis. The PI was located on the posterior part of the PD, between the dorsal side of PD and the neurohypophysis. The neurohypophysis consists only of the pars nervosa (PN).
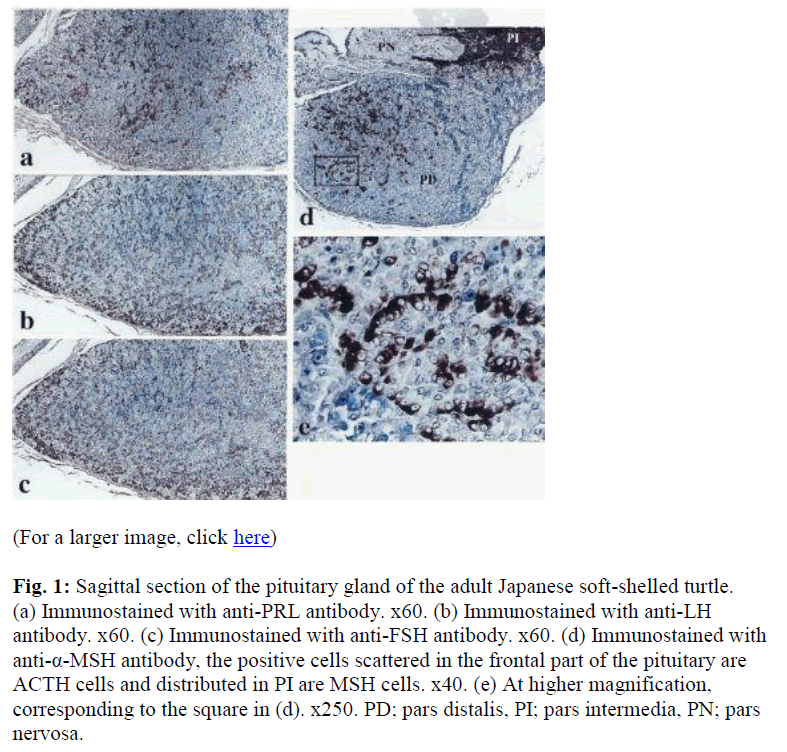
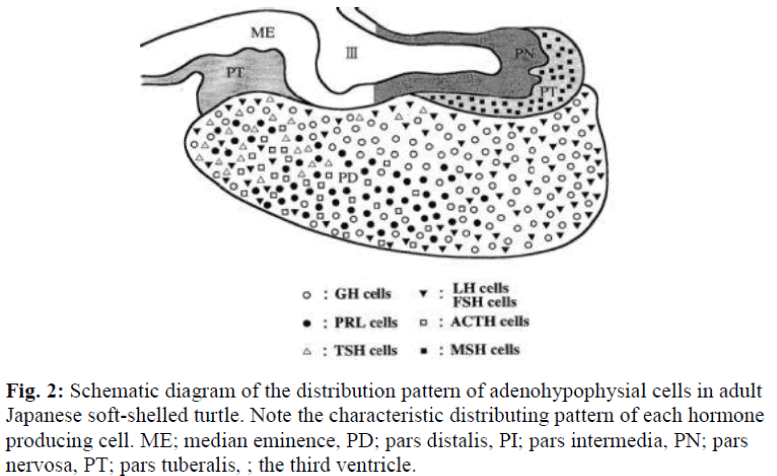
Immunohistochemically, seven distinct types of glandular cells (PRL, GH, TSH, LH, FSH, ACTH, and MSH) were identified in the adenohypophysis of adult Japanese soft- shelled turtles. The localization patterns of these cells are specific to each cell type. Cells which immunoreacted with the anti-PRL antibody were distributed extensively in the anterior and ventral part of the PD (Fig. 1a). A rostral area and the central area of the anterior PD were mainly occupied by ir-TSH cells and ir-ACTH cells, respectively. Part of the immunoreactive-ACTH cells were arranged regularly (Fig 1e). Immunoreactive-GH cells were distributed in the posterior part of the PD. Immuno-reactive-LH and ir-FSH cells were widely found in the PD, especially rostroventral to the posterior part of the PD (Figs 1b and c). Almost all immunoreactivity against anti LH and FSH antibodies were demonstrated in the same cells. In the PI, one cell type, ir-MSH cells, was detected. Immunoreactive-MSH cells were widely distri- buted in the PI (Fig. 1d). These results are summarized schematically in Figure 2.
Fig. 1: Sagittal section of the pituitary gland of the adult Japanese soft-shelled turtle. (a) Immunostained with anti-PRL antibody. x60. (b) Immunostained with anti-LH antibody. x60. (c) Immunostained with anti-FSH antibody. x60. (d) Immunostained with anti-α-MSH antibody, the positive cells scattered in the frontal part of the pituitary are ACTH cells and distributed in PI are MSH cells. x40. (e) At higher magnification, corresponding to the square in (d). x250. PD; pars distalis, PI; pars intermedia, PN; pars nervosa.
Fig. 2: Schematic diagram of the distribution pattern of adenohypophysial cells in adult Japanese soft-shelled turtle. Note the characteristic distributing pattern of each hormone producing cell. ME; median eminence, PD; pars distalis, PI; pars intermedia, PN; pars nervosa, PT; pars tuberalis, ; the third ventricle.
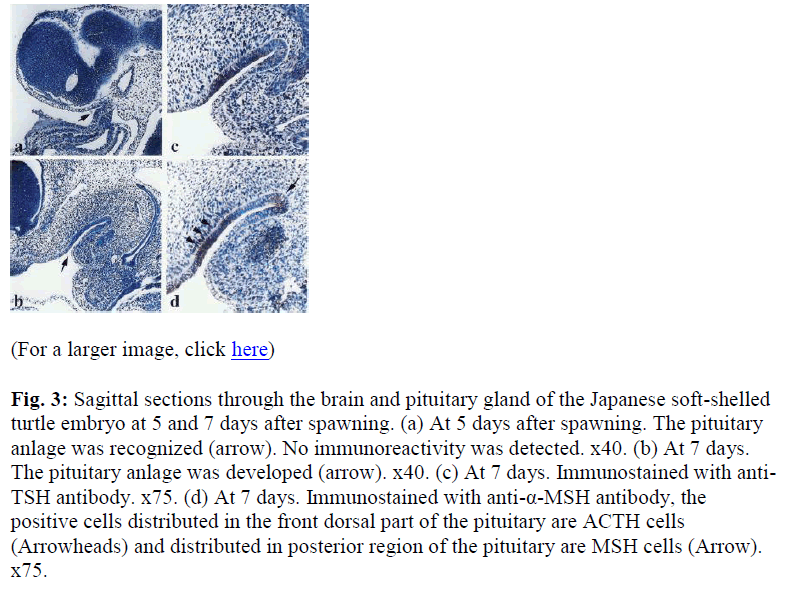
During embryonic development, five days after spawning, the adenohypophysial anlage was first recognized in the ventral region of the diencephalon. At this stage, the adenohypophysial anlage was a shallow hollow which consisted of the stomodeal epithelium at the dorsal oral cavity (Fig. 3a). There were no clear boundaries between the PD and PI in the adenohypophysis. No immunoreactivity was recognized in the pituitary anlage in this stage.
Fig. 3: Sagittal sections through the brain and pituitary gland of the Japanese soft-shelled turtle embryo at 5 and 7 days after spawning. (a) At 5 days after spawning. The pituitary anlage was recognized (arrow). No immunoreactivity was detected. x40. (b) At 7 days. The pituitary anlage was developed (arrow). x40. (c) At 7 days. Immunostained with anti-TSH antibody. x75. (d) At 7 days. Immunostained with anti-α-MSH antibody, the positive cells distributed in the front dorsal part of the pituitary are ACTH cells (Arrowheads) and distributed in posterior region of the pituitary are MSH cells (Arrow). x75.
Nine days after spawning, the adenohypophysial anlage became deeper and more elongated. The caudal region of the dorsal wall of the anlage folded ventrally, formed an acute angle, and led to the ventral wall of anlage (Fig.3b). At this stage, four types of immunoreactive cells, ir-TSH, LH, ACTH, and MSH cells were identified. Immunoreactive-TSH cells were demonstrated in the dorsal wall of the adenohypophysial anlage (Fig 3c).
Near the ir-TSH cells, a few ir-LH cells appeared. Therefore, the anti-ACTH antibody used in this study cross reacted with MSH, immunoreactive-ACTH cells and ir-MSH cells demonstrated in the same specimen. Immunoreactive-ACTH cells were detected on two thirds of the rostrodorsal wall of the anlage. Immunoreactive- MSH cells were distributed on the most caudal region of the anlage which was going to differentiate into the PI (Fig. 3d).
At 13 days after spawning, the anlage was more elongated, and a few ir-FSH cells were demonstrated in the dorsal wall.
At 22 days after spawning, the adenohypophysial anlage folded complicatedly, and formed the small embryonic adenohypophysis. At this stage, ir-GH and ir-PRL were scattered randomly in the embryonic adenohypophysis.
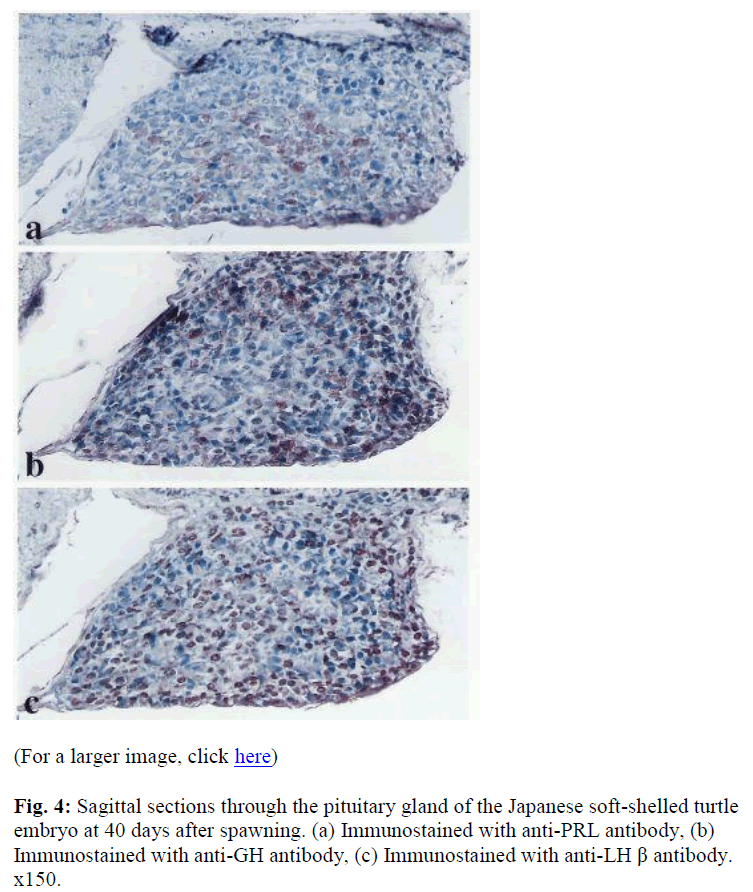
At 40 days after spawning, about five to seven days before hatching, the embryonic adenohypophysis had developed, and had almost the same shape as the adult adenohypophysis. At this stage, all adenohypophysial cells had differentiated. Immunoreactive PRL cells were mainly distributed in the centro ventral region of the PD (Fig. 4a). Immunoreactive GH cells, which were dem onstrated in the dorsal, ventral, and proximal region of the PD, where surrounding the area of ir-PRL cells (Fig. 4b). Immunoreactive-TSH cells and ACTH cells were scattered randomly in the rostral and central area of the PD, respectively. Immunoreactive-LH and ir-FSH cells were widely found in the PD (Fig. 4c). Almost all of immunoreactivity against anti-LH and FSH antibodies was demonstrated in the same cells as seen in the adult. Immunoreactive-MSH cells were distributed over the entire PI.
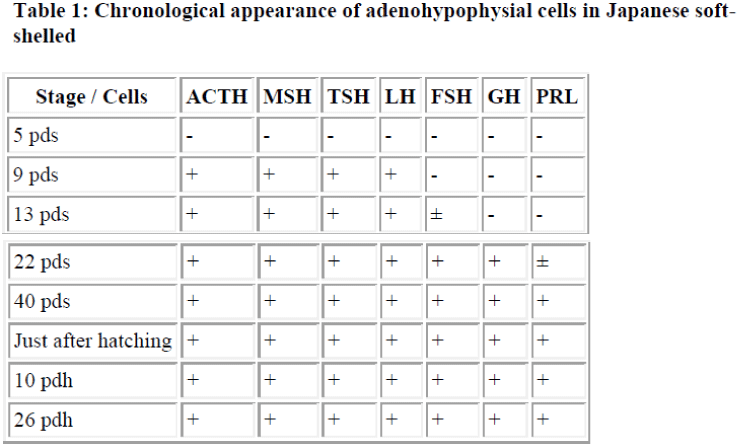
At 45 to 47 days after spawning, the Japanese soft-shelled turtle hatched. The adenohypophysis had developed fully, and the distributing pattern of each type of hormone-producing cell was shown similar to that of adults. These results are summarized in Table 1.
Other vertebrate adenohypophysial cells development
In fish, we studied four species of teleosts: two species of fresh water teleosts; rainbow trout (Oncorhynchus mykiss irideus) [3] and Nile tilapia (Oreochromis niloticus) [4], one species of brackish water teleost; ayu (Plecoglossus altivelis) [5], and one species of seawater teleost; Japanese whiting (Sillago japonica) [6].
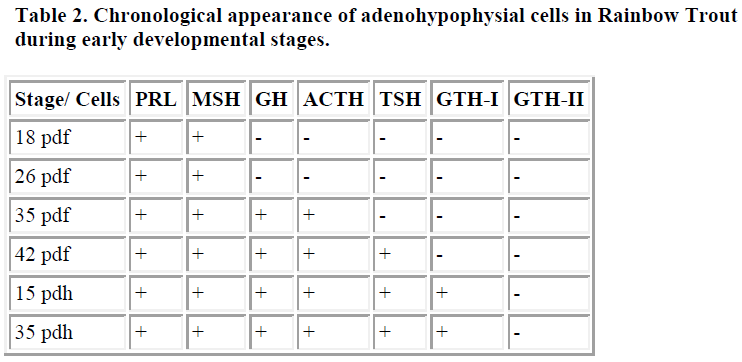
In rainbow trout (Oncorhynchus mykiss irideus), which spawns and grows in fresh water, the pituitary anlage was first recognized 18 days after fertilization. At this stage, ir-PRL and ir-MSH cells were identified in the rostral and caudal regions of the pituitary, respectively. After that, ir-GH, ir-ACTH, and ir-SL cells appeared 35 days after fertilization. In the final stage of before hatching (42 days after fertilization) ir-TSH cells were identified. Immuno-reactive GTH I cells appeared in the pituitary 15 days after hatching. Cells containing ir-GTH II were not apparent in the pituitary at any stage of embryonic or larval development, and were not present in pituitaries of trout at 6 months of age [3]. These results are summarized in Table 2.
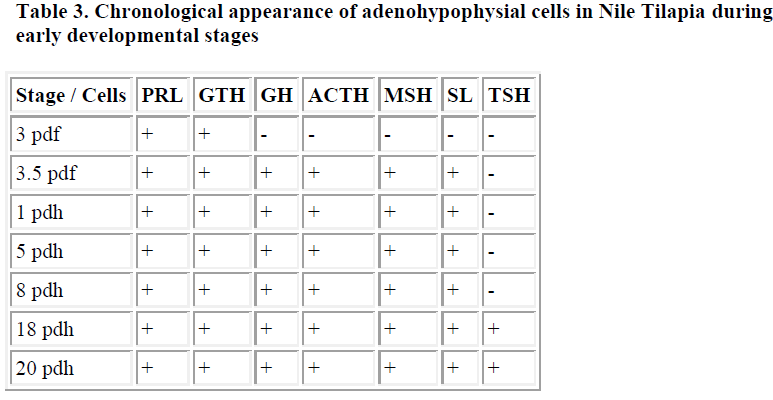
In Nile tilapia (Oreochromis niloticus), which spawns and grows in fresh water, the pituitary anlage was recognized in the ventral part of the third ventricle 3 days after fertilization. In this stage, two types of immunoreactive cells were recognized; some ir-PRL cells appeared in the antero ventral part of the pituitary and a few ir-GTH cells distributed in the centro dorsal region just above the ir-PRL cells. Just prior to hatching, 3.5 days after fertilization, four new types of immunoreactive cells were recognized; a small number of ir-GH cells appeared in the postero dorsal region of the pituitary, a small number of ACTH-ir cells existed in the antero dorsal part of the pituitary, some ir-MSH cells in the postero ventral region of the pituitary, and a few ir-SL cells on the ventral side of the ir-GTH cells. 18 days after hatching, a few ir-TSH cells appeared in the central part of the pituitary [4]. These results are summarized in Table 3.
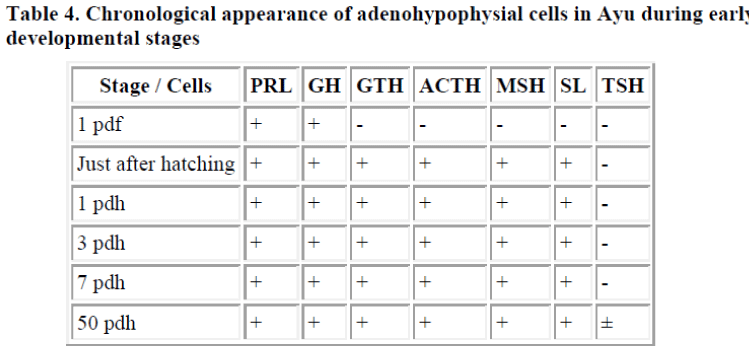
In ayu (Plecoglossus altivelis), which spawns and has its early development in brackish water, a few ir-PRL and ir-GH cells appeared in the ventral side of the pituitary one day before hatching. Subsequently, just after hatching, ir-GTH cells were recognized in the central to dorsal portion of the pituitary, ir-ACTH cells distributed in the anterior portion, and some ir-MSH and a few ir-SL cells in the posterior potion of the pituitary. Finally, a small number of ir-TSH cells appeared 50 days after hatching [5]. These results are summarized in Table 4.
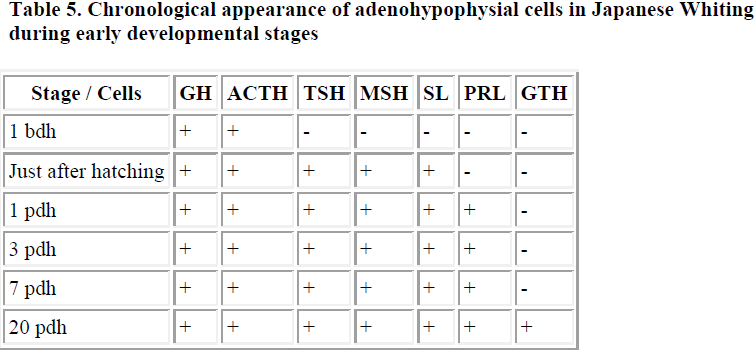
In Japanese whiting (Sillago japonica), which spawns and grows in seawater, a few ir-GH and ACTH cells were observed in the pituitary one day before hatching. Then, just after hatching, ir-TSH, MSH and SL cells appeared. Subsequently, one day after hatching, a few ir- PRL cells were demonstrated in the pituitary. Finally, a small number of ir-GTH cells were identified 20 days after hatching [6]. These results are summarized in Table 5.
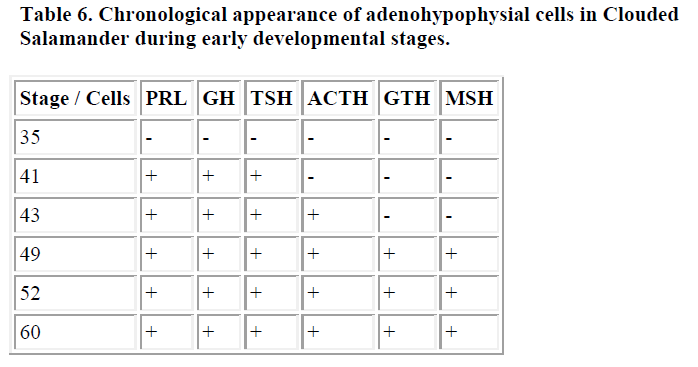
In amphibians, we studied one species of salamander called the Clouded salamander (Hynobius nebulosus) [7]. Immunoreactive-PRL, GH and TSH cells appeared in the embryonic pituitary before hatching. Then, just after hatching, ir-ACTH cells were demonstrated in the PD. Finally, ir-GTH cells were identified in PD, and ir-MSH cells were recognized in the PI [7]. These results are summarized in Table 6.
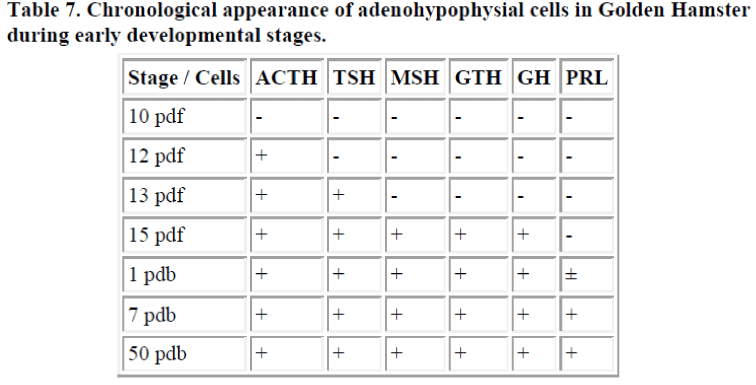
In mammals, the golden hamster (Mesocricetus auratus) was studied [8]. Immunoreactive-ACTH cell first appeared at 12 days gestation, after which ir-TSH cell differentiated at 13 days gestation. At 15 days gestation, just before birth, ir-MSH, GTH, and GH cells differentiated, and lastly ir-PRL cells were observed 1 day after birth [8]. These results are summarized in the table 7.
Discussion
There have been no previous immunocytochemical investigations concerning the distribution patterns of the various adenohypophysial hormone producing cells in the adult Japanese soft-shelled turtle (Pelodiscus sinensis japonicus). This is the first study to clarify the distribution patterns of these cells in the adult Japanese soft-shelled turtle immunocytochemically. In the adenohypophysis of the Japanese soft-shelled turtle, 7 distinct types of glandular cells (PRL, GH, TSH, LH, FSH, ACTH and MSH cells) were revealed. PRL cells were distributed in the anterior and ventral area of the pars distalis (PD), while most GH cells were distributed in the posterior area of the PD. TSH and ACTH cells were distributed in the rostral and central regions of the PD. LH and FSH cells were widely distributed in the PD, especially in the rostro ventral to posterior part of the PD. Almost all immunoreactivity against anti-LH and FSH antibodies was demonstrated in the same cells from embryo to adult PD. These LH/FSH cells were called gonadotroph (GTH) cells, and it is well known most of tetrapods have this type of GTH cells. MSH cells were widely distributed in the pars intermedia. These results were practically similar to the result Yip and Lofts [39], who reported the adenohypophysial cell distribution in the soft-shelled turtle (Trionyx sinensis) using conventional staining methods. In comparison with the other turtle, the localization patterns of adenohypophysial cells of the Japanese soft-shelled turtle were similar to those of the sea turtle (Caretta caretta) reported using immunocyto-chemical techniques [16]).
This is the first report on the chronological appearance of all different adenohypophysial hormone-producing cells of the Japanese soft-shelled turtle. Five days after spawning, the first cells to appear were TSH, LH, ACTH, and MSH cells. Subsequently, 13 days after spawning, a few FSH cells were observed. Next, 22 days after spawning, GH and PRL cells appeared. Regarding to other reptile species, only one immunocytochemical report was available on the chronological appearance of the adenohypophysial cells in the sea turtle (Caretta caretta) [16]. In this study it was reported that ACTH and MSH immuno reactive cells were first detected at an early developmental stage, followed by LH cells, lastly GH, PRL, and TSH cells appeared. Though the period to hatching of the Japanese soft- shelled turtle (about 43- 45 days) and sea turtle (about 65 days later) is different, all adenohypophysial hormone-producing cells of both species differentiated in a period half the total hatching period. Moreover, though the chronological order of adenohypophysial cells differs a little between the Japanese soft-shelled turtle and sea turtle, the early appearance of ACTH and MSH cells and late appearance of GH and PRL cells is similar in these two species. The physiological role of the early appearance of ACTH and MSH in the embryo is not well known. However, embryonic GH and PRL may primarily play an important role in growth, the turtle embryo has a large yolk in the egg and is rich to nourishment, so GH and PRL cells appear later than the other hormone- producing cells. These results may reflect that inhabiting environment is different after hatching, but embryonic developpment occurs in a similar environment where in the egg spawn under ground. In other reptiles, there have been no immunocytochemical investigations concerning the chronological appearance of all different adenohypophysial hormone producing cells. Further investigations using other species of reptile are necessary to clarify the functional role of each hormone during embryonic development.
Chronological appearance of the adenohypophysial cells in the other vertebrates
Until now, we studied chronological appearance of the adenohypophysial hormone producing cells in seven species of vertebrate [3-8]. In other vertebrates, many studies have been reported on the chronological appearance of all or a part of adenohypophysial cells by using immunocyto-chemical staining methods [9-32]. We compared the chronological appearance of the adenohypophysial cells in each class of vertebrate and considered the significance of the appearing hormones on early developmental. And in spite of the different early development periods of each animal, the chronological order of the studied adenohypophysial cells of each animal is shown in Table 8.
In the teleost, many reports on the localization patterns of adenohypophysial cells were similar to those of the other teleosts previously reported [1,40-43]. Different from other vertebrate, it is known that the teleosts have three particular hormones, two gonadotropins (GTH-I and GTH-II) instead of LH and FSH in the tetrapods, and one more type of PI hormone somatolactin (SL). Nozaki et al. [44,45] demonstrated that GTH-I and GTH-II were found in distinctly different cells in the salmonid pituitary, and GTH-I expressed first in ontogeny, whereas GTH-II cells appeared in the rainbow trout pituitary after vitellogenesis and spermatogenesis ensued. Moreover, Naito and colleagues [46] confirmed and supplemented these findings using in situ hybridization with probes for mRNA of the GTH-I and GTH-II beta subunits. In our previous study on the developing rainbow trout, we found that GTH-I cells were first detected shortly after hatching, whereas GTH-II cells were not detected throughout embryonic or larval development. These results extended previous data on the ontogeny of gonadotrophs to encompass all developmental and reproductive stages of rainbow trout. On the other hand, SL is a fish specific pituitary hormone which is belongs to the GH/PRL family. Therefore, in the fish PI, two types of hormones (MSH and SL) exist. Many cyto-chemical and physiological studies of the SL cells have been reported (see Ono and Kawauchi, for review [47]). Several reports have been published concerning the distribution of SL cells in the other teleosts [48,49]. Our results of the localization pattern of the SL cells in the adult fishes were similar to those of other teleosts previously reported [48,49]. However, ontogenetically, only several studies have been performed on SL cell differentiation [12,50,51].
Because the teleosts can be classified in three groups by spawning and inhabiting environment (freshwater teleosts, brackish water teleosts and seawater teleosts), we compared each group.
At present, there are many reports concerning the ontogeny of a part of adenohypophysial cells, only several reports are available on the chronological appearance of most of the adenohypophysial hormones producing cells in freshwater teleosts [3,4,9,11,12]. Mal et al. [9] reported that during the embryonic development of coho salmon (Oncorhynchus kisutch), PRL cells appeared first, followed by TSH, MSH and ACTH cells. GH and GTH-I cells appeared shortly after hatching, whereas GTH-II cells were not apparent at any stage of embryonic or larval development. Naito et al. [11] revealed the ontogeny of the pituitary cell types and the hypothalamo-hypophysial relationship during early development of chum salmon (Oncorhynchus keta). Their results were in good accordance with the results obtained from coho salmon [9] and rainbow trout [3]. In the rainbow trout [3], PRL and MSH cells were first identified 18 days after fertilization. Then GH and ACTH cells appeared 35 days after fertilization. During the last stage before hatching (42 days after fertilization) TSH cells appeared. GTH-I cells were observed 15 days after hatching, but GTH-II cells were not apparent at any stage of embryonic or larval development. In the Nile tilapia [4], at 3 days after fertilization, PRL and GTH cells were first recognized. Just before hatching, 3.5 days after fertilization, GH, ACTH, MSH and SL cells appeared. Lastly, TSH cells were detected 18 days after hatching. Recently, in the American shad (Alosa sapidis-sima) [12], PRL, ACTH and SL cells firstly detected 3 days after fertilization (3 days before hatching), then MSH cells appeared one day before hatching, and lastly GH and GTH cells were demonstrated at just hatching. They reported TSH cells were not detected in the American shad embryo and larvae until 42 days after hatching.
Regarding brackish water teleosts, only one our previous study was available on the chronological appearance of the adenohypophysial cells [5]. In the ayu (Plecoglossus altivelis), on one day before hatching, a few PRL and GH cells appeared. Then, just after hatching, GTH, ACTH, MSH and SL cells were recognized. Finally, TSH cells appeared 50 days after hatching [5].
Regarding marine teleosts, only one report was available on the chronological appearance of adenohypophysial cells [10] except for our report on Japanese whiting [6]. Cambré [10] reported in the sea bass (Dicentrarachus labrax) that GH, ACTH, TSH and MSH immunoreactive cells were already apparent on the first day after hatching, whereas PRL cells appeared between 9 and 15 days after hatching, and GTH cells could not be recognized during the first 26 days after hatching. We previously reported in Japanese whiting [6], GH and ACTH cells were observed one day before hatching. Then, just after hatching, TSH, MSH and SL cells appeared. Subsequently, PRL cells were demonstrated. Finally, GTH cells were identified 20 days after hatching.
Especially in fish, it is well known that GH and PRL play a very important osmoregulatory role in hyperosmotic and hypo-osmotic environments, respectively [52,53,54]. Thus, the moment of the appearance of PRL cells in the developing teleost pituitary varies considerably per species. The early appearance of PRL cells in freshwater teleosts may indicate that PRL plays an important role in osmoregulation of the embryo in these species of fishes [52]. In contrast, many reports have demonstrated that GH plays an important role in seawater adaptation [53,54]. Therefore, the early appearance of GH in seawater teleosts indicates that GH plays an important role in osmoregulation in seawater teleosts. Moreover, the early appearance of both GH and PRL in brackish water showed both characteristics in freshwater and seawater teleosts, so brackish water teleosts are able to adapt to their environment. Though the appearance order of the other hormones is slightly differ in the species, the tendency of the late appearance of GTHs and TSH cells was common. This suggests that GTHs and TSH are not involved in normal embryonic development.
In the amphibian, Moriceau-Hay, et al. [13] reported the appearance of TSH, LH and ACTH cells in the adeno-hypophysis of the tadpole Xenopus laevis. They showed ACTH cells were first demonstrated in the PD at stage 39, then the appearance of LH and TSH cells was confirmed, whereas they did not investigate PRL and GH cells. In Ambystoma gracile, Eagleson and McKeown [14] demonstrated three kinds of hormone-producing cells in the larval pituitary. They first showed the appearance of PRL cells, then TSH cells and lastly GTH cells in the pituitary until metamorphosis. In Rana dalmatina, Guastalla et al., [15] reported the appearance of PRL and GH cells in the early developmental stages; at stage 27, GH cells were first observed in the adenohypophysis, then at stage 28, PRL cells appeared. We previous reported on the immunohistochemical differentiation of the pituitary cells in the Clouded salamander, Hynobius nebulosus [7]. According to this report, at stage 41 (before hatching), PRL, GH and TSH cells were first detected, whereas in this stage all PRL and GH immunoreactivity was present in the same cells. Then PRL/GH cells may differentiate into two types of cells producing the GH or PRL hormone, respectively. This observation is in accordance with the report that only one type of an acidophilic cell has been demonstrated first in the PD of the amphibian larvae [55]. Next, at stage 43 (after hatching), ACTH cells appeared. At stage 49, FSH and LH cells were detected in the PD, and MSH cells were demonstrated in the PI. In amphibians, the tendency of the early appearance of PRL and GH cells was demonstrated as shown in the brackish water teleosts. Moreover, the relatively early appearance of TSH was shown in amphibian. This result may reflect that, in amphibians, TSH plays an important role during the metamorphosis.
In aves, Józsa et al. [17] only demonstrated differentiation of the embryonic chicken pituitary gland concerning PRL, GH and ACTH cells. PRL cells appeared first on the 6th day of incubation; ACTH cells were first found on the 7th day of incubation, lastly GH cells were observed in the pituitary on 12th day of incubation. However, Malamed et al. [18] reported the appearance of only GH cells in a 12 days old embryo, corresponding with the results of Józsa et al. [17]. Mikami [18] reported principally that LH cells were first observed on the fourth day of incubation, after which TSH and ACTH cells were found on the seventh day of incubation. Subsequently, on the eighth day of incubation, GH and FSH cells appeared, and lastly PRL cells were detected on the 9th day of incubation. In aves, though only a few reports are available, the chronological order of the appearance of the adenohypophysial cells is similar to that in reptiles; early appearance of LH and late appearance of PRL. These results may reflect that avian and reptilian animals are comparatively close on the phylogenetic tree.
In mammals, there have been reports relating to humans [20,21]. Bugnon et al. [20] showed ACTH cells were observed from the 8th fetal week and GH cells were revealed from the 9th fetal week. Subsequently, TSH cells appeared from the 13th fetal week. Lastly, LH or FSH cells were detected from the 15th fetal week. But they reported no PRL cells. Ikeda et al. [21] reported several ACTH cells and a few TSH cells appeared first in the pituitary primordium at the 9th fetal week. Then at the 13th fetal week, GH and FSH cells were demonstrated. Lastly, PRL cells and LH cells could be seen in the pituitary at the 21st fetal week. Many studies have been performed on rats [22-29]. Daikoku [26] especially reported on the chronological appearance of the pituitary hormone-producing cell in the rat. His results showed ACTH cells appeared first in the pituitary 14.5 days after gestation. Then TSH cells (16.5 days), LH cells (17.5 days), FSH cells, GH cells (19.5 days), and PRL cells (21.5 days) were detected. In the hamster, the appearance of a part of the adenohypophysial cells was reported except for our reports [30,31,32]. Mikami et al. [31] reported that ACTH, LH and TSH cells first appeared in the pars distalis at day 12.5 of gestation, then FSH cells appeared at day 14 of gestation. GH cells differentiated at day 15 of gestation (just before birth), whereas they did not observe the differentiation of PRL and MSH cells. We reported in the golden hamster that ACTH cell first appeared at 12 days gestation, and TSH cell differentiated at 13 days gestation. At 15 days gestation, just before birth, GGTH and MSH cells differentiated and lastly PRL cells were observed 1 day after birth [8]. In all species of mammal, the appearance of ACTH cells first and the appearance of PRL cells last has been reported. This early appearing ACTH may be required to survive the intrauterine development. On the other hand, the late appearance of PRL suggests that PRL in the mammal embryo is not involved in normal early development.
Generally, the order of appearance of PRL and GH cells varies greatly between species. The early differentiation of PRL cells was demonstrated in freshwater teleosts, brackish water teleost and amphibians. In contrast, the late appearance of PRL cells was reported in reptiles, aves and mammals. The tendency toward the late appearance of PRL cells was demonstrated in the amniotic animals and marine water teleosts. The early appearance of PRL in fresh water animals, such as the freshwater teleosts, brackish water teleosts and amphibians as described above, indicated that PRL may play an important role in osmoregulation in animals living in fresh or brackish water. On the other hand, the early differentiation of GH cells was shown in seawater and brackish water teleosts, and the slightly late (not last) appearance of GH cells was shown in other animals. GH has two important roles in seawater adaptation [53,54] and growth–promoting. The results indicate that early appearance of GH plays a role in the osmoregulation in seawater and brackish water teleosts. Moreover, the slightly late appearance of GH has another role in growth–promoting during the early developmental stages. Further study is required to clarify the functional significance of the early expression of PRL and GH, as well as of other hormones produced in the vertebrate embryo.
In conclusion, this study shows the chronological appearance of adenohypophysis in the Japanese soft-shelled turtle (Pelodiscus sinensis japonicus) and six other vertebrate species (rainbow trout, Nile tilapia, Ayu, Japanese whiting, clouded salamander and golden hamster). Comparisons with other animals investigated by other researchers, showed that each animal had a species-specific order of the appearance of the adenohypophysial cells and suggested the order was related to developmental style, spawning and inhabiting environment of each animal. The hormones appearing during the early developmental stages may be required to survive in each species-specific environment.
Acknowledgements
We would like to thank the late professor Dr. Mitsuaki Yoshizuka, our mentor, for his kind and unlimited guidance.
References
- DoerrSchott J. Immunohistochemical detection, by light and electron microscopy, of pituitary hormones in coldblooded vertebrates 1. Fish and amphibians. Gen Comp Endocrinol 1976; 28: 487512.
- DoerrSchott J. Immunohistochemical detection, by light and electron microscopy, of pituitary hormones in coldblooded vertebrates II. Reptiles. Gen Comp Endocrinol 1976; 28: 513-529.
- Saga T, Oota Y, Nozaki M, Swanson P. Salmonid pituitary gonadotrophs. III. Chronological Appearance of GTH I and other adenohypophysial hormones in the pituitary of the developing Rainbow Trout (Oncorhyn-chus mykiss irideus). Gen Comp Endocrinol 1993; 92: 233-241.
- Saga T, Adachi S, Yamauchi K, Kagawa H, Tanaka, Takahashi H. Immunocytochemical investigations of GTH cells in the pituitary of Nile tilapia (Oreochromis niloticus) during the embryonic development. Zool Sci 1992; 9: 1257.
- Saga T, Yamaki K, Doi Y, Yoshizuka M. Chronological study of the appearance of adenohypophysial cells in the ayu (Plecoglossus altivelis). Anat Embryol 1999; 200:469-476.
- Saga T, Aida K, Yamaki K, Yoshizuka M. Chronological study of the appearance of adenohypophysial cells in the Japanese whiting (Sillago japonica). Acta Anat Nippon 1996; 72: 339. (In Japanese).
- Saga T, Oota Y. Immunohistochemical differentiation of the pituitary cells in the salamander, Hynobius nebulosus. Rep Fac Sci Shizuoka Univ 1990; 24: 55-63.
- Saga T, Aida K, Yamaki K, Yoshizuka M. Chronological study of the appearance of adenohypophysial cells in the golden hamster (Mesocricetus auratus). Acta Anat Nippon 1996; 71: 477. (In Japanese)
- Mal AO, Swanson P, Dickhoff WW. Immunocyto-chemistry of the developing salmon pituitary gland. Amer Zool 1989; 29: 94A.
- Cambré M, Mareels G, Corneillie S, Moons L, Ollevier F, Vandesande F. Chronological appearance of the different hypophysial hormones in the pituitary of sea bass larvae (Dicentrarchus labrax) during their early development: An immunocytochemical demonstration. Gen Comp Endocrinol 1990; 77: 408-415.
- Naito N, de Jesus EG, Nakai Y, Hirano T. Ontogeny of pituitary cell-types and the hypothalamo-hypophysial relationship during early development of chum salmon, Oncorhynchus keta. Cell Tissue Res 1993; 272: 429- 437.
- Lais-Carrión R, Segura-Nogura MM, del Río MPM, Mancera JM. Ontogeny of adenohypophysial cells in the pituitary of the American shad (Alosa sapidissima). Gen Comp Endocrinol 2003; 132: 454-464.
- Moriceau-Hay D, Doerr-Schott J, Dubois, MP. Immunohistochemical demonstration of TSH-, LH- and ACTH-cells in the hypophysis of tadpoles of Xenopus laevis D. Cell Tissue Res 1982; 225: 57-64.
- Eagleson GW, McKeown BA. Localization of the pituitary lactotropes and thyrotropes within Ambystoma gracile by histochemical and immunochemical methods. A developmental study of two populations. Cell Tissue Res 1978; 189: 53-66.
- Guastalla A, Campantico E, Yamamoto K, Kobayashi T, Kikuyama S. Immunocytochemical and ultrastructural study of Rana dalmatina PRL and GH pituitary cells during larval development. Gen Comp Endocirnol 1993; 89: 364-377.
- Pearson AK, Wurst GZ, Cadle JE. Ontogeny and immunocytochemical differentiation of the pituitary gland in a sea turtle, Caretta caretta. Anat Embryol 1983; 167: 13-37.
- Józsa R, Scanes CG, Vigh S, Mess B. Functional differentiation of the embryonic chicken pituitary gland studied by immunohistological approach. Gen Comp Endocrinol 1979; 39: 158-163.
- Malamed S, Gibney JA, Cain LD, Perez FM, Scanes CG. Immunocytochemical studies of chicken somatotrophs and somatotroph granules before and after hatching. Cell Tissue Res 199; 272: 369-374.
- Mikami S. Immunocytochemistry of the avian hypothalamus and adenohypophysis. Int Rev Cytol 1986; 103: 189-248.
- Bugnon C, Lenys D, Bloch B, Fellmann D. Exploration cyto-immunologique sur coupes semi-fines des phénomènes de différenciation précoce de diverses populations cellulaires dans l’adénohypophyse foetale humaine. C R Soc Biol 1976; 170: 975-982.
- Ikeda H, Suzuki J, Sasano N, Niizuma H. The development and morphogenesis of the human pituitary gland. Anat Embryol 1988; 178: 327-336.
- Tougard C, Picart R, Tixier-VA. Cytogenesis of immunoreactive gonadotropic cells in the fetal rat pituitary at light and electron microscope levels. Dev Biol 1977; 58: 148-163.
- Watanabe YG, Daikoku S. An immunohistochemical study on the cytogenesis of adenohypophysial cells in fetal rats. Dev Biol 1979; 68: 557-567.
- Yashiro T, Nogami H, Yoshimura F. Immunohisto-chemical study of the postnatal development of pituitary thyrotrophs in the rat, with special reference to culster formation. Cell Tissue Res. 1981; 216: 39-46.
- Shirasawa N, Yoshimura, F. Immunohistochemical and electron microscopical studies of mitotic adenohypophysial cells in different