Research Article - Current Pediatric Research (2021) Volume 25, Issue 3
A comparative study for the efficacy of lactoferrin-100 versus lactoferrin-100 and ferrous gluconate versus ferric hydroxide on iron deficiency anemia.
Maha Youssef Kamal1*, Rezk MM2, Hafez MH1
1 Department of Pediatrics, University of Alexandria, Alexandria, Egypt
2 Department of Clinical Pathology, University of Alexandria, Alexandria, Egypt
- Corresponding Author:
- Maha Youssef Kamal Department of Pediatrics University of Alexandria Alexandria, Egypt
Tel: 00201223106023
E-mail: elsayedamr@yahoo.com
Accepted date: March 17th, 2021
Abstract
Objective: To estimate the effect of therapeutic doses of Lactoferrin 100, Lactoferrin 100 and ferrous gluconate compound, and Ferric Hydroxide polymaltose on the outcome of Iron Deficiency Anemia (IDA) in pediatric age group.
Methods: 150 children aged above 2 years suffering from IDA were recruited from the Outpatient Clinic of Alexandria University Children's Hospital. Children with confirmed IDA randomly allocated into three subgroups, (50 children each), Group A were treated by Lactoferrin 100, one packet for 2 times daily, Group B were treated by Lactoferrin 100 and ferrous gluconate compound, one packet for 2 times daily, Group C treated by Ferric hydroxide polymaltose 6 mg/kg/day 3 times daily, all for 3 months after diagnosis.
Results: There was no significant difference in the baseline levels of Hemoglobin (Hb), serum ferritin, serum iron (Fe), and total iron binding capacity among the three groups. In each group, all the biochemical indices were significantly improved when compared with baseline levels after 1.5 and 3 months of treatment (p=0.0001), but not for the mean of Fe in group A (Lactoferrin 100). There was no significant difference throughout the 3 month of treatment (p=0.700). There was a significant difference among the three groups as regards levels of Hb, iron and ferritin at 1.5 and 3 months of treatment, being the highest in the Lactoferrin 100 and ferrous gluconate compound group(B) (p=0.0001) followed by Ferric hydroxide polymaltose group(C).
Conclusion: Lactoferrin with iron may be considered as a more effective alternative treatment than traditional iron salt preparations for treatment of IDA.
Abstract
Objective: To estimate the effect of therapeutic doses of Lactoferrin 100, Lactoferrin 100 and ferrous gluconate compound, and Ferric Hydroxide polymaltose on the outcome of Iron Deficiency Anemia (IDA) in pediatric age group.
Methods: 150 children aged above 2 years suffering from IDA were recruited from the Outpatient Clinic of Alexandria University Children's Hospital. Children with confirmed IDA randomly allocated into three subgroups, (50 children each), Group A were treated by Lactoferrin 100, one packet for 2 times daily, Group B were treated by Lactoferrin 100 and ferrous gluconate compound, one packet for 2 times daily, Group C treated by Ferric hydroxide polymaltose 6 mg/kg/day 3 times daily, all for 3 months after diagnosis.
Results: There was no significant difference in the baseline levels of Hemoglobin (Hb), serum ferritin, serum iron (Fe), and total iron binding capacity among the three groups. In each group, all the biochemical indices were significantly improved when compared with baseline levels after 1.5 and 3 months of treatment (p=0.0001), but not for the mean of Fe in group A (Lactoferrin 100). There was no significant difference throughout the 3 month of treatment (p=0.700). There was a significant difference among the three groups as regards levels of Hb, iron and ferritin at 1.5 and 3 months of treatment, being the highest in the Lactoferrin 100 and ferrous gluconate compound group(B) (p=0.0001) followed by Ferric hydroxide polymaltose group(C).
Conclusion: Lactoferrin with iron may be considered as a more effective alternative treatment than traditional iron salt preparations for treatment of IDA.
Keywords
Iron deficiency anemia, Lactoferrin, Ferrous gluconate.
Introduction
Iron Deficiency Anemia (IDA) remains a major nutritional problem in developing countries particularly in children and is an important cause of morbidity. Microcytic anemia due to iron deficiency is the most common type of anemia in children. The U.S prevalence of iron deficiency anemia in children from one to five years of age is estimated to be 1%-2% [1].
Anemia is the most prevalent nutritional disorder among children in the Middle East and North Africa region [2]. The prevalence of anemia among Egyptian children and adolescents reaches 39.6% [3]. Austin et al. examined the trends in anemia (Hemoglobin (Hb) <11 g/dL) from the Egyptian Demographic and Health Survey (EDHS) conducted between 2000 and 2005, and revealed a prevalence of 37%-52% among Egyptian children aged 12-36 months. This rise in the prevalence of anemia was attributed to shifting food consumption patterns and increases in childhood diarrhea.
According to the EDHS 2014 report, IDA appears to be declining [4]. Nonetheless, more than one in four young children in Egypt suffers from some degree of anemia. Moreover, the proportion of children <5 years with any anemia in the 2014 EDHS at 27.2% was similar to the level reported in 444 the 2000 EDHS at 30.3%, but considerably lower than the level reported in the 2005 EDHS at 48.5%. Girls aged 5-19 years were slightly more likely to be anemic than boys in this age group (21% and 18%, respectively) [5,6].
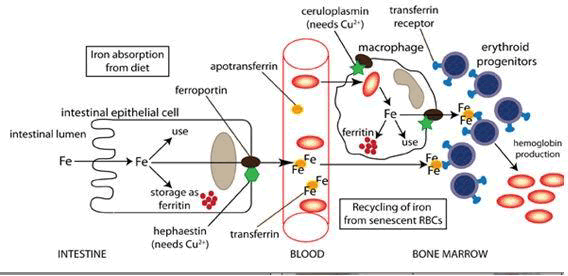
People lose a small but steady amount of iron by sweating and by shedding cells of the skin and the mucosal lining of the Gastro Intestinal Tract (GIT). This steady loss means that people must continue to absorb iron; they do so via a tightly regulated process as Figure 1 shows [7,8].
In human cells, the required iron is guaranteed by Transferrin (Tf) bound iron, which is imported into cells through receptor- mediated endocytosis which is translocated into cytoplasm where it is sequestered by Ferritin (Ftn). The release of iron from this protein to cytoplasm occurs by Ferroportin (Fpn), the only known mammalian iron exporter found on the cytoplasmic membrane of enterocytes, hepatocytes, macrophages and placental cells [8]. Fpn is an important actor of iron homeostasis, regulated by multiple factors. In particular, Fpn is down-regulated by the pro-inflammatory cytokine interleukin-6 (IL-6) [9,10]. And by hepcidin, another pivotal actor, which regulates iron homeostasis through the binding, internalization and degradation of Fpn [11].
The bioactive hepcidin is a cationic peptide hormone of 25 amino acids mainly synthesized by hepatocytes [12]. As Fpn, hepcidin is controlled by several factors. In particular, it is transcriptionally feedback-regulated by iron stores [13]. This mechanism involves multiple pathways through which hepatocytes directly sense systemic iron levels [13,14]. Hepcidin synthesis is also up-regulated by pro-inflammatory cytokines, such as IL-6, IL-α1_ and IL-β1 [15-18].
The Fpn degradation caused by the binding with hepcidin or its down-regulation by IL-6 provokes a significant decrease of iron export from cells into plasma. Consequently, at the cellular level, iron overload in the host cells including enterocytes and macrophages is established, while at the systemic level, Iron Deficiency (ID), Iron Deficiency Anemia (IDA), and Anemia of Inflammation (AI) have been found [19]. Lactoferrin (LF), discovered in 1939 in bovine milk and extracted in 1960 from both human and bovine milk. LF is one of the most important glycoprotein in iron homeostasis owing to its multifunctional activities.
Lactoferrin is a member of the transferrin family of iron binding glycoproteins. Human Lactoferrin (HLF) is a glycoprotein formed of 691 amino acids, constitutively expressed and secreted by glandular epithelial cells, and by neutrophils following induction [20,21]. Standard oral iron therapy consists of provision of 3-6 mg/kg/day of elemental iron in 2-3 divided doses for 1-2 months to correct the anemia, followed by an additional 2-3 months of therapy to increase hepatic iron stores [22].
Subjects and Methods
This Prospective study was conducted on 150 children aged above 2 years suffering from IDA, attending to the Outpatient Clinic of Alexandria University Children's Hospital.
Inclusion criteria
Proved Iron deficiency anemia by decreased serum ferritin, decreased serum iron and decreased hemoglobin.
Exclusion criteria
Chronic anemias, chronic diseases, acute or chronic infections, and chronic blood loss. Patients were subjected to thorough history taking and clinical examination. Blood samples from all studied individuals were obtained through venipuncture, under complete aseptic technique. Laboratory work-up included Complete Blood Count (CBC) with (CHr) using Ethylenediaminetetraacetic Acid (EDTA)-blood specimen with the Siemens ADVIA 2120 analyzer, occult blood in the stool, C-Reactive Protein CRP (mg/L) and iron profile including serum iron (μg/L), Total Iron Binding Capacity TIBC (μg/L) and serum ferritin (ng/mL). Among children with confirmed IDA 150 subjects were randomly selected after fulfilling the inclusion criteria and subsequently were assigned into 3 subgroups, (50 children in each group).
Group-A: Treated by administration of lactoferrin 100 one packet 2 times daily for three months after diagnosis.
Group-B: Treated by administration of lactoferrin 100 and ferrous gluconate compounds, one packet 2 times daily for three months after diagnosis.
Group-C: Treated by administration of Ferric hydroxide 6 mg/kg/day three times daily for three months after diagnosis.
After administration of drugs, the studied cases were followed up in 0, 1.5 and 3 months using CBC and serum iron profile (Fe, Ferritin, TIBC).
Results
There were no significant differences in the baseline levels of Hb, iron, TIBC and ferritin among the 3 groups (p>0.05) as shown in Table 1.
| Lactoferrin 100 | lactoferrin 100 and ferrous gluconate | Ferric hydroxide | Test of significance | |
|---|---|---|---|---|
| (n=50) | (n=50) | (n=50) | (p) | |
| Hemoglobin (Hb) | 8.2 ± 0.97 | 8.4 ± 0.83 | 8.6 ± 0.8 | F=1.777 |
| p=0.173 NS | ||||
| Serum iron (Fe) | 0.32 ± 0.96 | 0.22 ± 0.07 | 0.18 ± 0.07 | F=0.892 |
| p=0.412 NS | ||||
| TIBC | 7.2 ± 1.7 | 6.9 ± 1.9 | 7.0 ± 1.3 | F=0.416 |
| p=0.661 NS | ||||
| Ferritin | 4.1 ± 1.2 | 6.0 ± 5.1 | 5.7 ± 7.2 | F=1.902 |
| p=0.153 NS | ||||
| Data in means ± SD | ||||
| F: F ratio of analysis of variance | ||||
| NS: Not Statistically significant |
Table 1: Baseline measurement in the three studied groups.
There was a significant difference among the 3 groups according to the levels of Hb, iron and ferritin in 1.5 month period of treatment being the highest in the lactoferrin 100 and ferrous gluconate compound group (B). (p=0.0001*) followed by Ferric hydroxide polymaltose group(C). As regard the TIBC, there were significant differences among the 3 groups. It was lowest in the lactoferrin 100 and ferrous gluconate compound group (B) followed by Ferric hydroxide polymaltose group (C) (p=0.0001*) as shown in Table 2.
| Lactoferrin 100 | lactoferrin 100 and ferrous gluconate | Ferric hydroxide | Test of significance | |
|---|---|---|---|---|
| (n=50) | (n=50) | (n=50) | (p) | |
| Hemoglobin (Hb) | 8.9a ± 0.99 | 10.4b ± 0.79 | 9.5c ± 0.87 | F=34.4 |
| p=0.0001* | ||||
| Serum iron (Fe) | 0.23a,c ± 0.06 | 0.27b ± 0.05 | 0.24a,c ± 0.05 | F=7.1 |
| p=0.001* | ||||
| TIBC | 6.7a,c ± 1.5 | 5.1b ± 0.92 | 6.1a,c ± 1.35 | F=17.9 |
| p=0.0001* | ||||
| Ferritin | 5.2a ± 1.3 | 8.1b,c ± 1.4 | 7.7b,c ± 4.3 | F=9.5 |
| p=0.001* | ||||
| Data in means ± SD | ||||
| F: F ratio of analysis of variance | ||||
| Different superscript letters indicates statistically significant difference using post-hoc multiple comparison by Bonferroni method. | ||||
| * : Statistically significant (p<0.05) |
Table 2: Comparison of studied parameters after 1.5 month of treatment among the three studied groups.
There were significant differences among the 3 groups according to the levels of Hb, iron and ferritin in 3 month period of treatment, being the highest in the lactoferrin 100 and ferrous gluconate compound group (B) (p=0.0001*) followed by Ferric hydroxide polymaltose group (C).
As regard the TIBC, there were significant differences among the 3 groups. It was lowest in the lactoferrin 100 and ferrous gluconate compound group (B) followed by Ferric hydroxide polymaltose group (C) (p=0.0001*).
According to the levels of Hb between the group (B) and (C), it was higher in the group (B), but without a statistically significant difference (P2=0.146) as shown in Table 3.
| Lactoferrin 100 | lactoferrin 100 and ferrous gluconate | Ferric hydroxide | Test of significance | |
|---|---|---|---|---|
| (n=50) | (n=50) | (n=50) | (p) | |
| Hemoglobin (Hb) | 9.7a ± 1.2 | 11.6b,c ± 0.7 | 11.2b,c ± 0.9 | F=45.3 |
| P=0.0001* | ||||
| Serum iron (Fe) | 0.32a,b ± 0.18 | 0.41a,b,c ± 0.29 | 0.38b,c ± 0.12 | F=18.00 |
| P=0.0001* | ||||
| TIBC | 5.3a,c ± 1.5 | 4.1b ± 0.8 | 4.9a,c ± 1.2 | F=18.3 |
| P=0.0001* | ||||
| Ferritin | 7.7a ± 3.6 | 19.6b ± 4.45 | 11.4c ± 4.16 | F=3.7 |
| P=0.026* | ||||
| Data in means ± SD | ||||
| F: F ratio of analysis of variance | ||||
| Different superscript letters indicates statistically significant difference using post-hoc multiple comparison by Bonferroni method. | ||||
| * : Statistically significant (p<0.05) |
Table 3: Comparison of studied parameters after 3 months of treatment among the three studied groups.
Discussion
Anemia is a public health problem affecting both developing and developed countries all over the world. According to WHO, globally, it affects 1.62 billion people which correspond to 24.8% of the population with the highest prevalence among infants (6-24 months) (47.4% all over the world and 67.6% in Africa) [23]. Iron deficiency is a typical prevalent disease in Egypt. It influences all age groups from all demographic strata.
Oral iron supplementation is well-accepted and time-tested mode of treatment of iron deficiency anemia in all age groups except very few cases of gastrointestinal intolerability [24].
However, poor compliance and prolonged duration of treatment are limiting factors in effective management of this problem. A joint UNICEF/USAID consultation has recommended that the most practical iron supplement for use in infancy and childhood should be an aqueous solution of a soluble ferrous salt, such as Ferrous Sulphate (FS) or a ferric complex, such as Iron Polymaltose (IPC) [25]. Both of them have been demonstrated to have equivalent bioavailability in infants [26].
The results of the study are similar to Chen et al. and Ke et al. who reported that bLF fortification to iron-fortified formula for 3 months had a beneficial effect on improving Hb levels and decreasing the prevalence of anemia when compared with nonfortification (iron-fortified formula only) [27,28]. These results were hypothesized to be due to the effects of bLF fortification on iron metabolic homeostasis that were mainly in the context of iron absorption in the intestine. The two studies were conducted on normal infant’s ages 4-6 months. Another study done by Chierici et al. proposed that bovine lactoferrin significantly induced higher serum ferritin levels compared to the unsupplemented formula at day 90 and day 150 [29]. These observations favour the idea that lactoferrin may be involved in iron absorption.
In support of the present study’s results, another study was done by Mostafa et al. in the department of Pediatrics, Alexandria University, Egypt to compare the efficacy of ferric hydroxide polymaltose to that of lactoferrin with ferrous gluconate (compound group) on normal children aged from 2 years up to 6 years and their compliance to both. The findings of this study demonstrated that there was a significant rise in mean Hb, SI, and SF in children who had taken lactoferrin with ferrous gluconate compared to children who had taken iron polymaltose group with less adverse effects as regards constipation, nausea, vomiting and abdominal pain [30]. In 2006, similar results were met among pregnant females in a clinical trial conducted by Paesano et al. on the effect of 30 days of bLf oral administration (100 mg two times a day before meals) in pregnant women with IDA or anemia of inflammation (AI), surprising results were obtained [31]. In fact, pregnant women receiving 100 mg of bLf, iron saturated at 20-30% two times a day acquired 70-84 μg/day of iron, respectively. Although the concentration of iron supplemented by bLf is very far from that which is required daily (1-2 mg), a significant increase of the concentration of Hb and SI was detected after 30 days which presumably was not linked to direct iron supplementation, but to a more complex mechanism involving this protein in iron homeostasis. In other clinical trials such as Paesano et al., Rateb et al. and Rezk et al., bLf treatment showed a significant improvement of hematological parameters, including red blood cells number, hemoglobin, total serum iron, serum ferritin concentrations and percentage of hematocrit over other oral iron preparations with less side effects in pregnant and non-pregnant women suffering from IDA [32-34].
Paesano et al. study explained the previous results (by measuring IL6) [32]. They found that, in pregnant women, bLf decreased serum IL-6 (P<0.0001), and increased prohepcidin (precursor of hepcidin). However oral iron preparations like Ferrous sulfate increased IL-6 (P<0.0001) and decreased prohepcidin (P=0.093). They had strong evidence that this effect could be due to the capacity of bLf to lower serum IL-6, which in turn mitigates ferroportin down regulation, thus permitting iron export from tissues to blood and restoring physiological values of total serum iron and serum ferritin, thus curing ID and IDA. The parallel increase of serum prohepcidin (or hepcidin) is related to an increase of hematological parameters.
Regarding the effect of ferric hydroxide polymaltose on improving IDA, a similar study was conducted in the Department of Paediatrics at Rajendra Institute of Medical Sciences (RIMS), where 50 children were enrolled, 26 of which were assigned to the ferrous sulphate group, and 24 were assigned to the ferric hydroxide polymaltose group. In the ferric hydroxide polymaltose group, 66.67% (16 of 24 patients) achieved Hb>11 g/dL, which was the primary end point 3.52 ± 0.42 g/dL in this group. After treatment with iron preparations for 8 weeks, Hb was also measured at 2, 4, and 6 weeks [35].
Another study was conducted by Ozsurekci et al, to compare the efficacy and compliance of different iron formulation. Iron polymaltose containing preparations was found to be effective like ferrous sulphate preparations [36]. Ferrous iron preparations were found to have more side-effects than ferric preparations (IPC). Also Geisser published a review of over 25 years’ experience on iron polymaltose in terms of acceptance and patient compliance, IPC clearly had an advantage over ferrous salts [37]. Furthermore Toblii and Brignoli reported in a meta-analysis that both ferrous sulfate and IPC seemed to be associated with a similar hemoglobin level at outcome, but IPC was more tolerable than ferrous sulfate [38].
Most researchers agree that iron is absorbed better from breast milk. In part, this may be due to the presence of high concentrations of the iron-binding protein lactoferrin in breast milk and its virtual absence from infant formula [39]. A major part of iron in breast milk is associated with lactoferrin. Human lactoferrin is absorbed across the apical membrane of the intestinal cell by a specific lactoferrin receptor and internalized with its bound iron. Thus, lactoferrin facilitates a unique mechanism for absorption of iron from breast milk [40]. Lactoferrin with iron may be considered as a more effective alternative treatment than traditional iron salt preparations as treatment of IDA. This was also demonstrated in Rateb et al. and Mostafa et al. studies [33,30]. which is also consistent with findings of the present study.
In contrast to the present results, a review published in 1994, where the two studies were conducted on term infants below 6 month of age and reported that no significant differences in hematology or iron status were observed between blf fortified group and non -fortified groups [41,42]. On the other hand, Lo¨nnerdal et al. found that infants’ capacity to regulate iron homeostasis is important but less well understood than the regulation of iron absorption in adults [43]. Infants who were given daily iron drops compared with a placebo from 4 to 6 month of age had similar increases in hemoglobin concentrations. In addition, isotope studies have shown no difference in iron absorption between infants with high or low hemoglobin concentrations at 6 month of age [44]. Together, these findings suggest a lack of homeostatic regulation of iron homeostasis in young infants which is largely due to a lack of regulation of the iron transporters and ferroportin. The high and up regulated absorption of iron in the newborn period may confer developmental benefits but raises the possibility of excessive iron accumulation [43]. Therefore, according to Brock et al study, the role of lactoferrin in iron absorption may be to regulate, rather than to promote, iron uptake from the gut to the circulation in early infancy (below 6 months of age) [45]. In particular, full term newborn are iron replete and do not need to absorb iron from milk at that stage where the milk lactoferrin concentrations are highest [46]. However, at 9 month of age, homeostatic regulatory capacity has developed although its extent is not known [44].
Conclusion
According to the results obtained by the present study, it can be concluded that iron deficiency in kids needs more powerful treatment modalities. Lactoferrin with ferrous gluconate is better over ferric hydroxide polymaltose complex, and lactoferrin 100mg in treatment of iron deficiency. Lactoferrin helps better absorption of iron.
Recommendations
According to the present study, we may suggest that treatment with lactoferrin with ferrous gluconate might be considered as a more powerful elective treatment than conventional iron salt arrangements. Further studies and researches are still needed for additional confirmation of these outcomes.
Declaration of Interest
The authors report no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Trial was registered and approved at Cochrane South Africa under the unique identification number: PACTR202010465119274.
All procedures performed in this study were in accordance with the institutional research committee's ethical standards of Faculty of Medicine, University of Alexandria, Egypt, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All participants in this study signed informed written consent approved by the local ethical committee at Alexandria Faculty of Medicine, Egypt.
References
- Wang M. Iron Deficiency and other types of anemia in infants and children. Am Fam Physician. 2016;93(4):270-8.
- Austin AM, Fawzi W, Hill AG. Anaemia among Egyptian children between 2000 and 2005: trends and predictors. Matern Child Nutr. 2012;8(4):522-32.
- Tawfik A, Hanna ET, Abdel-Maksoud AM. Anemia and iron deficiency anemia in Egypt. IOSR J Pharm. 2015;5:30-4.
- El Zanaty F WA. Egypt demographic health survey 2014. Ministry of health and population Cairo, Egypt, National Population Council. 2014.
- Mousa SMO, Saleh SM, Higazi AMM, et al. Iron deficiency and iron deficiency anemia in adolescent girls in rural upper Egypt. Int Blood Res Rev. 2016;5(4):1-6.
- Hwalla N, Al Dhaheri A, Radwan H, et al. The prevalence of micronutrient deficiencies and inadequacies in the Middle East and approaches to interventions. Nutrients. 2017;9(3):229.
- Borch-Iohnsen B, Hagve T, Hauge A, et al. Regulation of the iron metabolism. Tidsskr Nor Laegeforen. 2009;129(9):858-62.
- Donovan A, Lima CA, Pinkus JL, al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1(3):191-200.
- Cutone A, Frioni A, Berlutti F, et al. Lactoferrin prevents LPS-induced decrease of the iron exporter ferroportin in human monocytes/macrophages. Biometals. 2014;27(5):807-13.
- Cutone A, Rosa L, Lepanto MS, et al. Lactoferrin efficiently counteracts the inflammation-induced changes of the iron homeostasis system in macrophages. Front Immunol. 2017;8:705.
- Qiao B, Sugianto P, Fung E, et al. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012;15(6):918-24.
- Hunter HN, Fulton DB, Ganz T, Vogel HJ. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J Biol Chem. 2002;277(40):37597-603.
- Coffey R, Ganz T. Iron homeostasis: An anthropocentric perspective. J Biol Chem. 2017;292(31):12727-34.
- Zumerle S, Mathieu JR, Delga S, et al. Targeted disruption of hepcidin in the liver recapitulates the hemochromatotic phenotype. Blood. 2014;123(23):3646-50.
- Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clinical Invest. 2004;113(9):1271-6.
- Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204-9.
- Verga Falzacappa MV, Vujic Spasic M, Kessler R, et al. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353-8.
- Lee P, Peng H, Gelbart T, et al. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102(6):1906-10.
- Frazer DM, Anderson GJ. The orchestration of body iron intake: how and where do enterocytes receive their cues?. Blood Cells Mol Dis. 2003;30(3):288-97.
- Rosa L, Cutone A, Lepanto MS, et al. Lactoferrin: a natural glycoprotein involved in iron and inflammatory homeostasis. Inte J Mol Sci. 2017;18(9):1985.
- Karav S, German JB, Rouquié C, et al. Studying lactoferrin N-glycosylation. Int J Mol Sci. 2017;18(4):870.
- Powers JM, Buchanan GR. Diagnosis and management of iron deficiency anemia. Hematol Oncol Clin North Am. 2014;28(4):729-45.
- De Benoist B, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia 1993-2005. WHO global database on anaemia. 2008.
- Shah A. Iron deficiency anemia--Part III. Indian J of Med Sci. 2004;58(5):214-6.
- Nestel P, Alnwick D. Iron/multi-micronutrient supplements for young children. Summary and conclusions of a consultation held at UNICEF Copenhagen, Denmark. 1996;19-20.
- Borbolla JR, Cicero RE, Dibildox M, et al. Iron polymaltose complex vs iron sulfate for the treatment of iron deficiency anemia in infants. Revista Mexicana de Pediatria. 2000;67(2):63-7.
- Chen G, Chen T, Chen H, et al. Effect of lactoferrin fortified formula on infant growth and development and the accounts of peripheral blood cells. CJ Postgrad Med 2011;34:52-5.
- Ke C, Lan Z, Hua L, et al. Iron metabolism in infants: influence of bovine lactoferrin from iron-fortified formula. Nutrition. 2015;31(2):304-9.
- Chierici R, Sawatzki G, Tamisari L, Volpato S, Vigi V. Supplementation of an adapted formula with bovine lactoferrin. 2. Effects on serum iron, ferritin and zinc levels. Acta Paediatr. 1992;81(6‐7):475-9.
- El walili T, EL hadad R, Mostafa m. A Comparitive study of the efficacy and tolerability of iron polymaltose and ferrous gluconate with lactoferrin in treatment of iron deficiency anemia. Master Thesis, Alexandria University 2019.
- Paesano R, Torcia F, Berlutti F, et al. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem Cell Biol 2006;84(3):377-80.
- Paesano R, Berlutti F, Pietropaoli M, et al. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int J Immunopathol Pharmacol. 2010;23(2):577-87.
- Rateb AM, Mamdouh AM, Balsha KM. The effect of orally administered iron-saturated lactoferrin on systemic iron homeostasis in pregnant women suffering from iron deficiency and iron deficiency anaemia. Egypt J Hospitl Med 2018;71(4):2851-7.
- Rezk M, Dawood R, Abo-Elnasr M, et al. Lactoferrin versus ferrous sulphate for the treatment of iron deficiency anemia during pregnancy: A randomized clinical trial. J Maternal-Fetal Neonat Med. 2016;29(9):1387-90.
- Chaudhuri PK CA. Therapeutic efficacy of Iron polymaltose complex versus Ferrous sulphate in treatment of Iron deficiency anemia in children. IOSR J Dent Med Sci. 2017:16(1):27-9.
- Ozsurekci Y, Unal S, Cetin M, et al. Comparison of ferrous sulfate, polymaltose complex and iron-zinc in iron deficiency anemia. Minerva Pediatr. 2019;71(5):449-54.
- Geisser P. Safety and efficacy of iron (III)-hydroxide polymaltose complex. Arzneimittelforschung. 2007;57(6A):439-52.
- Toblli JE, Brignoli R. Iron (III)-hydroxide polymaltose complex in iron deficiency anemia. Arzneimittelforschung. 2007;57(6A):431-8.
- Lonnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93-110.
- Suzuki YA, Shin K, Lonnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001;40(51):15771-9.
- Lönnerdal B, Hernell O. Iron, zinc, copper and selenium status of breast‐fed infants and infants fed trace element fortified milk‐based infant formula. Acta Paediatr. 1994;83(4):367-73.
- Hernell O, Lönnerdal B. Iron status of infants fed low-iron formula: no effect of added bovine lactoferrin or nucleotides. Am J Clin Nutr. 2002;76(4):858-64.
- Lönnerdal B. Development of iron homeostasis in infants and young children. Am J Clin Nutr. 2017;106(Suppl 6):1575S-80S.
- Domellof M, Lonnerdal B, Abrams SA, et al. Iron absorption in breast-fed infants: effects of age, iron status, iron supplements, and complementary foods. Am J Clin Nutr. 2002;76(1):198-204.
- Brock J. Lactoferrin in human milk: its role in iron absorption and protection against enteric infection in the newborn infant. Arch Dis Child. 1980;55(6):417-21.
- Brock JH. Lactoferrin-50 years on. Biochem Cell Biol. 2012;90(3):245-51.