Case Report - Biomedical Research (2018) Volume 29, Issue 10
A case report of successful treatment of metastatic gastral neuroendocrine carcinoma to a small-molecule VEGFR-2 tyrosine kinase inhibitor apatinib
Li Nong and Yong-kui Lu*
Affiliated Cancer Hospital of Guangxi Medical University, PR China
- *Corresponding Author:
- Yong-kui Lu
Affiliated Cancer Hospital of Guangxi Medical University, PR China
Accepted on April 10, 2018 Biomed Res
DOI: 10.4066/biomedicalresearch.29-17-3677
Visit for more related articles at Biomedical ResearchAbstract
Context: Neuroendocrine Tumor (NET) is arising from cells throughout the diffuse endocrine system. They comprise a broad family of tumors. Neuroendocrine Carcinoma (NEC) is a poorly differentiated and high-grade type. Advanced NEC has a poor prognosis due to a limited efficacy for chemotherapy and radiotherapy.
Case Presentation: We present here a 51-year-old Chinese woman initially diagnosed with advanced gastral neuroendocrine carcinoma. She accepted everolimus as the first line treatment, but progression after 1 month. And then she received two different cytotoxic chemotherapy regimens. Unfortunately, the treatment failed. Then, she received apatinib, a novel tyrosine kinase inhibitor of vascular endothelial growth factor receptor-2 that has been used in the treatment of patients with metastatic gastric cancer who progressed with 2 or more chemotherapy regimens. This patient was partially responsive to apatinib with a dose of 500 mg daily. Tolerated drug-related side effects were observed.
Conclusion: Our findings indicate that some cases of neuroendocrine carcinoma may be responsive to antiangiogenic agent apatinib. Further large-scale prospective studies are needed to optimize the treatment.
Keywords
Apatinib, Gastral, Neuroendocrine carcinoma, Chemotherapy, Targeted therapy, Tyrosine kinase inhibitor, VEGFR-2.
Abbreviations
NET: Neuroendocrine Tumor; NEC: Neuroendocrine Carcinoma; ECOG: Eastern Cooperative Oncology Group; OS: Overall Survival; PFS: Progression-Free Survival; VEGFR-2: Vascular Endothelial Growth Factor Receptor-2; mTOR: Mammalian Target of Rapamycin.
Introduction
Neuroendocrine tumor is thought to arise from cells throughout the diffuse system. They comprise a broad family of tumors, Neuroendocrine Carcinoma (NEC) is a poorly differentiated and high-grade type (grade 3). The most common of which are carcinoid and pancreatic neuroendocrine tumor. An analysis of the SEER databases estimated that the incidence of neuroendocrine tumor in the United States was 5.25 cases per 100,000 people in the year 2004 [1]. Patients with neuroendocrine tumor may or may not have symptoms attributable to hormonal hyper secretion [2,3]. Standard treatment for early stage or no metastatic gastric NEC, endoscopic resection or surgical resection indicated [4]. Patients who have metastatic tumor and carcinoid syndrome should be treated with a somatostatin analog, but no clear consensus exists on the timing of octreotide initiation asymptomatic patients [5]. Everolimus is an inhibitor of mammalian target of rapamycin (mTOR) which can use in metastatic neuroendocrine tumors [6-8]. The tumor response rate is generally low and no PFS benefit to cytotoxic chemotherapy in advanced NET [9-11]. For advanced NET, the prognosis remains very poor, even with combined, multimodal therapy. There is an urgent need for novel effective agents.
Apatinib (Hengrui Pharmaceutical Co., Ltd, Shanghai, People’s Republic of China) is a novel oral small-molecule Tyrosine Kinase Inhibitor (TKI) targeting the intracellular domain of Vascular Endothelial Growth Factor Receptor-2 (VEGFR-2). Apatinib-mediated VEGFR-2 inhibition also appears to inhibit downstream phosphorylated extracellular signal-regulated kinase [12]. Through this inhibition, Apatinib plays antiangiogenic and antitumor roles. It has shown a survival benefit in gastric cancer in a phase III trial [13] and is currently being studied in multiple tumor types including metastatic lung, colon, and breast cancer.
Here, we report a case of a 51-year-old Chinese woman with advanced gastric NEC, who received apatinib after failure of everolimus and second-line chemotherapy. The patient got a more than 11 months PFS.
Case Presentation
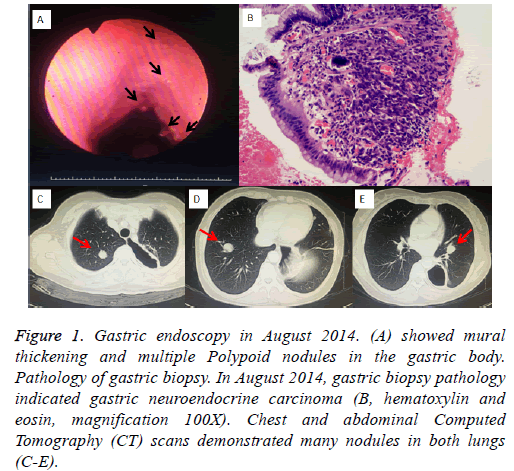
A 51-year-old Chinese woman diagnosed with gastral neuroendocrine carcinoma by gastroscope was referred to our hospital- Department of Chemotherapy, the cancer hospital of Guangxi medical University (Nanning, China) in August 2014 (Figure 1A). Pathology diagnosis confirmed NEC (Figure 1B) with immunophenotype: CKpan (-), Syn (+), CD56 (+), CD57 (+), S100 (+), NSE (-). Neither abdominal mass nor superficial lymph node was palpable. The patient had no fever, cough, hemoptysis and chest pain, had no carcinoid syndrome either. Chest and abdominal Computed Tomography (CT) scans demonstrated many nodules in both lungs (Figures 1C-1E).
Figure 1: Gastric endoscopy in August 2014. (A) showed mural thickening and multiple Polypoid nodules in the gastric body. Pathology of gastric biopsy. In August 2014, gastric biopsy pathology indicated gastric neuroendocrine carcinoma (B, hematoxylin and eosin, magnification 100X). Chest and abdominal Computed Tomography (CT) scans demonstrated many nodules in both lungs (C-E).
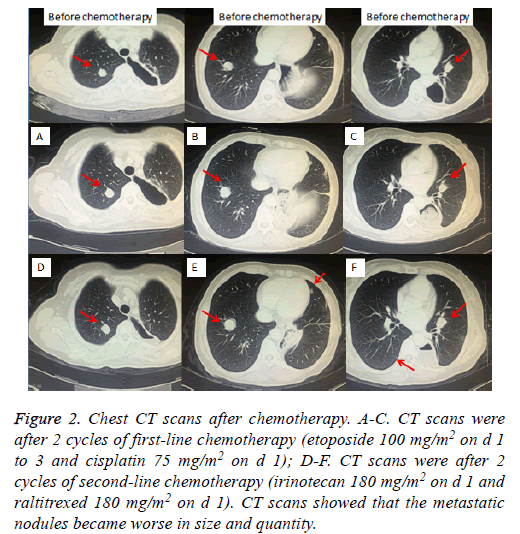
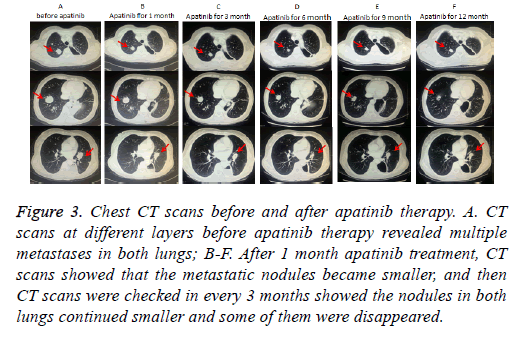
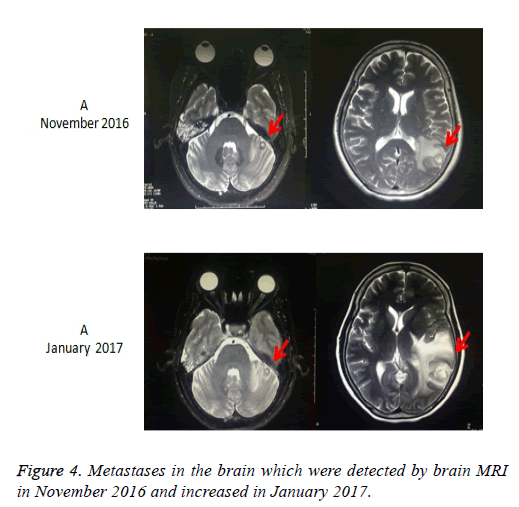
Taking into account the lung has a lot of metastatic nodules, surgery was not performed. Due to the NCCN guideline [14], we first performed the treatment with everolimus 10 mg per day. The chest CT after taking everolimus for 1 month revealed the nodules in both lungs increased. Then, the patient received 2 cycles of first-line palliative chemotherapy (etoposide 100 mg/m2 on d 1 to 3 and cisplatin 75 mg/m2 on d 1), repeated every 3 w. CT examination after the second cycle showed slight increasing of Metastatic tumors of lung, which indicated Stable Disease (SD) according to Response Evaluation Criteria in Solid Tumors (RECIST) [15] (Figures 2A-2C). After firstline chemotherapy, the patient was performed 2 cycles of second-line chemotherapy (irinotecan 180 mg/m2 on d 1 and raltitrexed 180 mg/m2 on d 1), repeated every 2 w. Unfortunetly, CT examination revealed Progressive Disease (PD) after two cycles chemotherapic treatments (Figures 2D-2F). The patient refused any treatment after second-line chemotherapy until January 2016, she came back to our hospital for tussiculation, CT showed the revealed an increased metastasis in both lungs. After the patient provided written, informed consent, apatinib was then administered with a dose of 500 mg/d on January 26, 2016. We evaluate the efficacy after 1 month of targeted therapy, CT showed Lung metastases were significantly reduced in size and quantity, which was considered to be a PR (Figures 3A-3F), moreover, symptoms of tussiculation reduced significantly. We continued following up the patient and check CT every 2 months, she maintained PR and No severe toxicities were observed (Table 1). The patient is considering whole brain radiotherapy due to metastases in the brain which were detected by brain MRI in November 2016 and increased in January 2017 (Figure 4). Nonetheless, she is now still undergoing the apatinib treatment on account of stable disease in Metastatic tumors of lung. A progression-free survival time of more than 11 months has been achieved.
Figure 2: Chest CT scans after chemotherapy. A-C. CT scans were after 2 cycles of first-line chemotherapy (etoposide 100 mg/m2 on d 1 to 3 and cisplatin 75 mg/m2 on d 1); D-F. CT scans were after 2 cycles of second-line chemotherapy (irinotecan 180 mg/m2 on d 1 and raltitrexed 180 mg/m2 on d 1). CT scans showed that the metastatic nodules became worse in size and quantity.
Figure 3: Chest CT scans before and after apatinib therapy. A. CT scans at different layers before apatinib therapy revealed multiple metastases in both lungs; B-F. After 1 month apatinib treatment, CT scans showed that the metastatic nodules became smaller, and then CT scans were checked in every 3 months showed the nodules in both lungs continued smaller and some of them were disappeared.
| Adverse event | Adverse reaction grade | After treatment |
|---|---|---|
| Hypertension | Grade II | Normal value |
| Hand-foot syndrome | Grade I | Disappear |
| Proteinuria | Grade II | Disappear |
| Neutropenia | Grade I | Normal value |
Table 1. Adverse events of apatinib.
Discussion
The benefits associated with cytotoxic chemotherapy in advanced NET appear to be modest, tumor response rate are generally low and no PFS benefit has been clearly demonstrated [16]. Capecitabine was used to treat advanced NET (19 cases) in a stage II study demonstrated no PR and 13 cases SD [9]. In another stage III study (E1281), which evaluated the combination of 5-FU with doxorubicin or streptozocin got a response rate of nearly 16% in both groups [10]. Everolimus, an inhibitor of mTOR is a choice to advanced NET. In a random phase III study (RADIANT-2), 429 patients were performed to two groups: group 1 (combination everolimus with octreotide) and group 2 (combination placebo with octreotide), and the everolimus group showed a PFS of 16.4 months to a PFS of 11.3 months of octreotide (P=0.026) [6]. In our case, the Chinese woman received everolimus and two lines chemotherapy, but failed.
Apatinib is an oral, highly potent tyrosine-kinase inhibitor targeting VEGFR2 [17]. Phases II and III studies of apatinib have shown exciting efficacy and good safety in Chinese gastric patients who have failed at least 2 chemotherapeutic regimens [13]. In the phase III study of apatinib, 5 273 patients were randomly assigned to oral apatinib group or placebo group at a ratio of 2:1. The results showed that patients receiving apatinib had significantly longer median overall survival (195 vs. 140 d; P<0.016) and longer median Progression Free Survival (PFS) (78 vs. 53 d; P<0.0001) compared with those receiving placebo. Both in the phases II and III studies of apatinib, the 3 most common adverse events were hypertension, hand-foot syndrome, and proteinuria. But there was no significant difference in severe adverse events. Several phase II clinical study was performed in other tumors, including advanced non-small cell lung cancer and advanced hepatocellular carcinoma [18], it demonstrated significantly prolonged in PFS. At present, clinical trials of apatinib include colorectal cancer, osteosarcoma and esophageal carcinoma.
Apatinib effectively inhibiting proliferation, migration and tube formation of human umbilical vein endothelial cells. Apatinib also inhibits the growth of tumors, either alone or in combination with chemotherapeutic drugs [19,20]. Importantly, cells experiment showed that apatinib can reverse Multidrug Resistance (MDR) by inhibits P-glycoprotein (ABCB1/MDR1) and ABCG2 (BCRP) which mediated transport function [21]. It also targets ABCB1-overexpressing leukemia cells HL-60, downregulates P-Erk1/2 to enhancing the efficacy of doxorubicin [22].
As for this case, the patient took apatinib after failures to everolimus and two lines chemotherapy. To our best knowledge, this is the first report of successfully using apatinib to treat advanced NET, whose best efficacy was PR, and obtained a PFS more than 11 months without severe adverse reaction. She possibly expressed MDR who could not benefit from chemotherapy, and we should check MDR before using apatinib. Are there biotical markers to pre-test the efficacy of apatinib? Is there a better prognosis in patients with high VEGFR-2 expression? We can collect tissue samples and expand the sample size of patients for VEGFR-2 mRNA detection in the future.
In conclusion, because of low side effects and improved outcomes, apatinib has demonstrated a substantial potential to be a new therapeutic option in advanced NET and a variety of tumor types.
References
- Yao JC, Hassan M, Phan A. One hundred years after carcinoid: epidemiology of and prognostic factors for neuroendocrine tumor in 35,825 cases in the United States. J Clin Oncol 2008; 26: 3063-3072.
- Jenson RT, Norton JA. Carcinoid tumor and caicinoid syndrome. Cancer: Principles and Practice of Oncology (6th Edn.). Philadelphia Pa: Lippincott Williams and Wilkins 2001; 1813-1826.
- Joynt KE, Moslehi JJ, Baughman KL. Paragangliomas: etiology, presentation, and management. Cardiol Rev 2009; 17: 159-164.
- Saund MS, AI Natour RH, Sharma AM. Tumor size and depth predict rate of lymph node metastasis and utilization of lymph node sampling in surgically managed gastric carcinoids. Ann Surg Oncol 2011; 18: 2828-2832.
- Oberg K, Kvols L, Caplin M. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 2004; 15: 966-973.
- Pavel ME, Hainsworth JD, Baudin E. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumors associated with cacinoid syndrome (RADIANT-2): a randomized placebo-controlled, phase 3 study. Lancet 2011: 1378: 2005-2012.
- Parithivel K, Ramaiya N, Jagannathan JP. Everolimus and temsirolimus associated enteritis: report of three cases. J Clin Oncol 2010; 29: 404-406.
- Yao JC, Phan AT, Chang DZ. Efficacy of evoerolimus and octreotide LAR in advanced low-to intermediate-grade meuroendocrine tumor: results of a phase II study. J Clin Oncol 2008; 26: 4311-4318.
- Medley L, Morel AN, Farrugia D. Phase II study of single agent capecitabine in the treatment of metastatic non-pancreatic neuroendocine tumours. Br J Cancer 2011; 104: 1067-1070.
- Sun W, Lipsitz S, Catalano P. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005; 23: 4897-4904.
- Bajetta E, Catena L, Procopio G. Are capecitabine and oxaliplatin (XELOX) suitable treatment for progressing low-grade and high-grade neuroendocine tumours? Cancer Chemother Pharmacol 2007; 59: 637-642.
- Haijun Z. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther 2015; 13: 6075-6081.
- Li J, Qin S, Xu J. Randomized, double-blind, placebo-controlled phase III Trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016; 34: 1448-1454.
- Shah MH, Lombard-Bohas C, Ito T. Everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET): Impact of somatostatin analog use on progression-free survival in the RADIANT-3 trial. J Clin Oncol 2011; 29: 4010.
- Nishino M, Jackman DM, Hatabu H. New response evaluation criteria in solid tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol 2010; 195: 221-228.
- Paulson AS, Bergsland EK. Systemic therapy for advanced carcinoid tumor: Where do we go from here? J Natl Compr Canc Netw 2012; 10: 785-793.
- Li J, Zhao X, Chen L, Guo H, Lv F, Jia K, Yv K, Wang F, Li C, Qian J, Zheng C, Zuo Y. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010; 10: 529.
- Qin SK. Apatinib in Chinese patients with advanced hepatocellular carcinoma: A phase II randomized, open-label trial. J Clin Oncol 2014; 32: 17-20.
- Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011; 102: 1374-1380.
- Chen P, Iruela-Arispe L, Lou L, Sun P, Yuan K. VEGFr inhibitor YN968D1 xenograft dose response studies against human colon cancer Ls174t and HT29. Proc Amer Assoc Cancer Res 2006; 47: 1764.
- Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, Ma XX, To KK, Ambudkar SV, Chen ZS, Fu LW. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010; 70: 7981-7991.
- Tong XZ, Wang F, Liang S, Zhang X, He JH, Chen XG, Liang YJ, Mi YJ, To KK, Fu LW. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol 2012; 83: 586-597.